Copy link
ABG measurement: Temp effect
Last updated: 05/02/2016
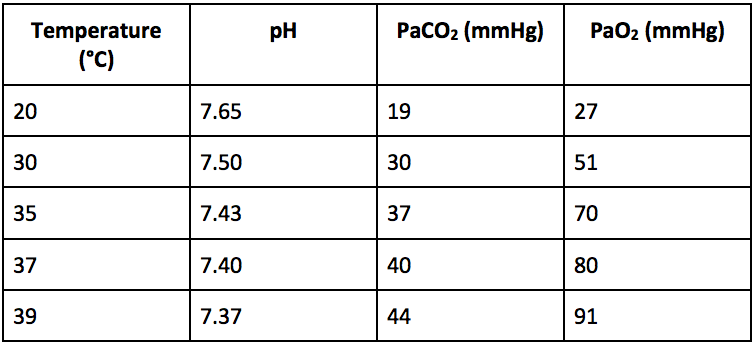
The electrodes in a blood gas analyzer are maintained at 37°C. Therefore, all ABG measurements are made at 37°C regardless of the patient’s body temperature. However, the solubility of a gas (CO2, O2) in liquid (blood) is inversely proportional to the temperature of the liquid. Therefore, when the patient’s body temperature is below 37°C the reported PaO2 and PaCO2 will be lower than the actual values in the patient (temperature decreases –> solubility increases –> partial pressure decreases), and the opposite is true when the patient’s body temperature is above 37°C. Likewise, pH is also temperature-dependent and decreases with increasing temperature. Normal values adjusted for temperature are shown in the table below.
Alternatively, corrected values can be estimated according to the following relationships:
Physiologic PO2 (mmHg) = Measured PO2 – 5 * [37°C – Body Temperature (°C)]
Physiologic PCO2 (mmHg) = Measured PCO2 – 2 * [37°C – Body Temperature (°C)]
Physiologic pH = Measured pH + 0.015 * [37°C – Body Temperature (°C)]
Other References
- Keys to the Cart: December 12, 2016; A 5-minute video review of ABA Keywords Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.