Copy link
Benzodiazepines
Last updated: 03/26/2024
Key Points
- Benzodiazepines (BZDs) act on the GABA-A receptor leading to increased inhibitory action on the CNS.
- BZDs act as anxiolytics, sedatives, anticonvulsants, and amnestics and are widely used for anxiety and sleep disorders, preoperative anxiolysis, acute alcohol withdrawal, and more.
- BZDs have a number of side effects, and vulnerable populations, such as the elderly, are the most susceptible.
- Substance use disorder is a serious risk of prolonged use, with an associated risk of BZD overdose and withdrawal.
Introduction
- All BZDs share a basic structure of a benzene ring joined to another seven-member ring named “diazepine” (Figure 1).
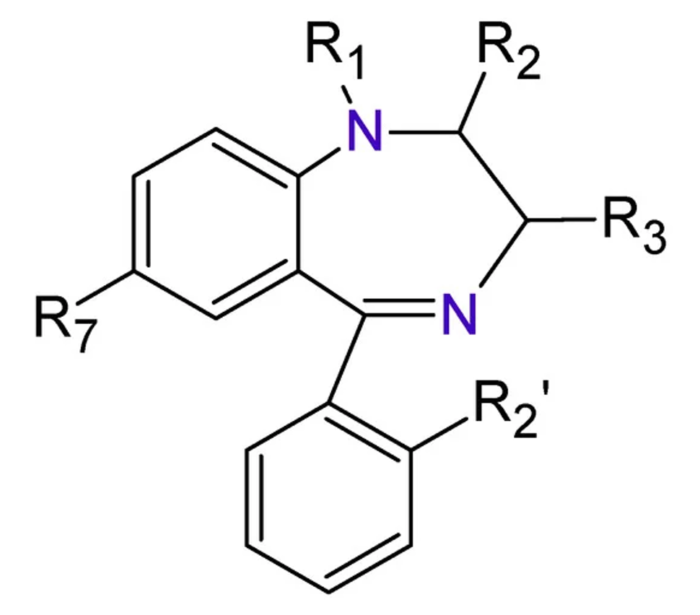
Figure 1. The base structure of BZD includes a benzene ring attached to a seven-member “diazepine” ring. Source: Sanabria E, et al. Toxics. 2021;9(2):25.1 CC BY
- Variations in functional groups branching off the core structure result in the number of BZDs on the market today (Figure 2).
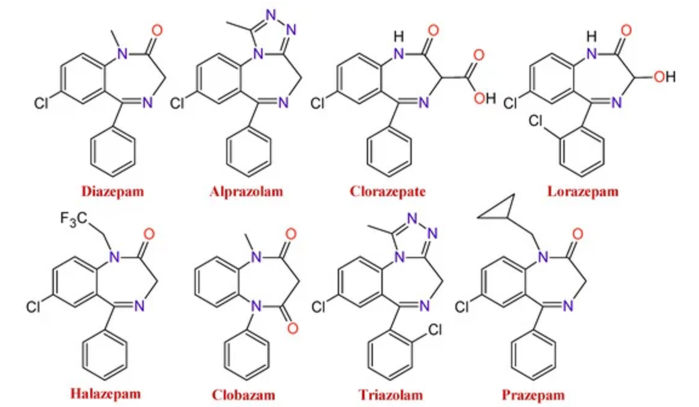
Figure 2. Changes to the functional groups produce several BZDs with different pharmacological properties. Source: Sanabria E, et al. Toxics. 2021;9(2):25.1 CC BY
Mechanism of Action
- All BZDs function as positive allosteric modulators of the gamma aminobutyric acid (GABA)-A receptor, a ligand-gated chloride ion channel.2
- GABA functions as the premier inhibitory neurotransmitter for the central nervous system (CNS). High levels of GABA act to relax and calm the brain.
- GABA-A receptor is a glycoprotein complex with 5 subunits (2 alpha, 2 beta, and 1 gamma). BZDs bind where the alpha and gamma subunits meet.2
- Binding of the BZD at the GABA-A receptor results in a conformational change that hyperpolarizes the cell and elicits the CNS inhibitory effect.
- There are two known BZD receptors – BZ1 and BZ2.
- BZ1 is found mostly in the cerebral cortex, thalamus, and cerebellum. Binding of the BZ1 receptor leads to sedation, anticonvulsive properties, and anterograde amnesia.
- BZ2 is found mostly in the limbic system, motor neurons, and dorsal horns. This subunit is responsible largely for the strong anxiolytic effect of BZDs. Some BZDs interact more strongly to one subunit over the other, resulting in varied effects.2
Pharmacokinetics
- BZDs can be administered orally, intramuscularly, intravenously, sublingually, intranasally, or rectally.2
- The volume of distribution varies between BZDs due to characteristics such as size, lipid solubility, and ability to bind to plasma proteins.
- The route of administration affects the absorption and speed of systemic onset. Administration varies based on indication and specific BZD.
- BZDs exhibit a high affinity for plasma proteins and bind strongly to albumin.
- They congregate in lipid-dense regions of the body, such as the CNS and adipose tissue. In general, the more lipophilic the BZDs, the more rapid the onset of effects.2
- BZDs are metabolized in the liver through microsomal oxidation or glucuronide conjugation. Microsomal oxidation is the primary pathway for the metabolism of diazepam and midazolam.
- Many long-acting BZDs, such as diazepam, will produce active metabolites that continue to exhibit pharmacologic effects. Other short-acting BZDs, such as midazolam, do not have active metabolites and wear off more rapidly.2
- BZDs are primarily eliminated via the kidneys.
Systemic Effects
- Clinically, BZDs are often categorized by the length of their half-lives.2
- Short-acting – median half-life of 1-12 hours
- Midazolam, triazolam
- Intermediate-acting – median half-life of 12-40 hours
- Alprazolam, lorazepam, oxazepam
- Long-acting – median half-life of 40-250 hours
- Chlordiazepoxide, clonazepam, diazepam3
- Cardiovascular effects
- BZDs act to decrease arterial blood pressure, cardiac output, and peripheral vascular resistance. Heart rate is occasionally increased.
- On their own, BZDs has minimal effects on left ventricular (LV) function , but when combined with opioids, they work synergistically and can lead to LV depression.
- Respiratory effects
- BZDs reduce the response to CO2. This effect is usually mild. Response is more pronounced with intravenous (IV) BZD administration or in combination with other respiratory depressants (opioids).
- IV doses of BZDs can cause apnea or respiratory arrest.
- Titration of IV BZDs and monitoring of ventilation is recommended for all patients receiving IV BZDs.
- Neurological effects
- While not as significant as barbiturates, BZDs do decrease cerebral oxygen use, blood flow, and intracranial pressure.
- Anxiolytic, amnestic, and sedative effects should be provided at low doses.
- High doses lead to unconsciousness.
- Unconsciousness is slower with BZDs than with other inductors, such as propofol. Recovery of BZD unconsciousness is also slower.5
Clinical Use
- Common indications for BZDs are listed in Table 1. This list is not exhaustive, and additional BZDs can be used for each indication.4-7
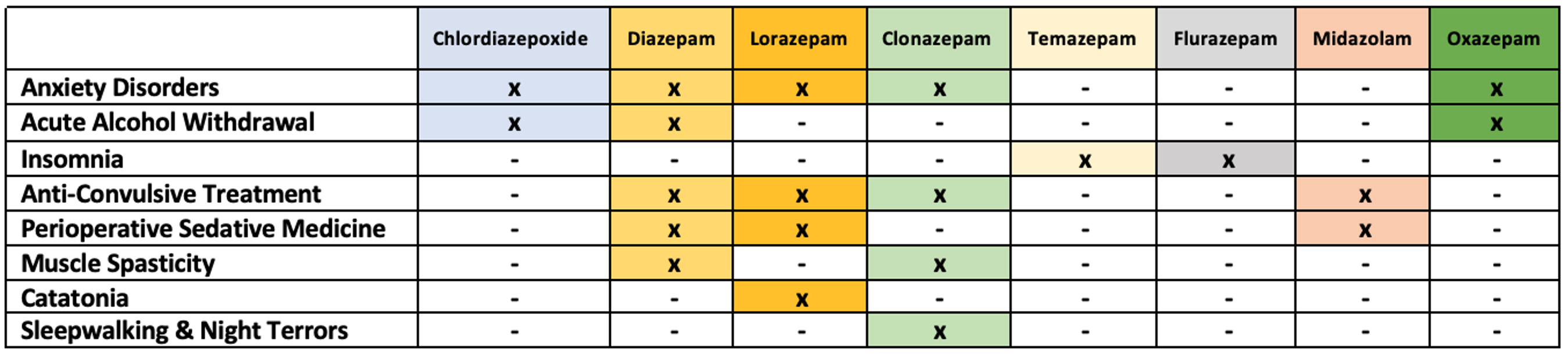
Table 1. Common indications for BZD
Side Effects
- Acute anterograde amnesia is a side effect of BZDs. This is often temporary and beneficial in the perioperative setting.4
- BZDs cause decreased motor coordination, cognitive impairment, and overall sedative effects. This is a major concern for elderly patients as the use of BZDs can lead to increased fall risk.6
- BZDs should be avoided in patients with myasthenia gravis, ataxia, angle-closure glaucoma, and chronic respiratory insufficiency.4
- Paradoxical reactions have been seen in <1% of patients taking BZD, and includes increased psychomotor symptoms, talkativeness, and excitement. Treatment is discontinuation of the BZD.8
- There is a risk of rebound tolerance and overdose with BZD use.4
- BZDs carry a high risk of substance use disorder.
Benzodiazepine Dependence
- Studies have shown that BZDs activate dopaminergic neurons in the ventral tegmental area, part of the mesolimbic section of the CNS that leads to addictive potential.4 This is similar to other drugs that carry a high risk of substance use disorder.
- Withdrawal symptoms of BZDs mirror the effects of alcohol withdrawal, with symptoms ranging from weakness, spasms, anxiety, and depression to acutely severe symptoms, including delirium, psychosis, and seizures.4
- Gradual discontinuation of BZDs over the course of several weeks is key to reducing the risk of severe withdrawal symptoms. Discontinuing BZDs over the course of 4-8 weeks has been shown to be sufficient in most patients.
- In settings of severe withdrawal, seizure prophylaxis as well as acute seizure management may be necessary.4
Benzodiazepine Overdose
- BZD overdose is a life-threatening condition due to the CNS and respiratory depression that can occur.
- It is commonly seen in patients who are taking BZDs in combination with other substances that cause CNS and respiratory depression, such as alcohol and opioids.
- CNS symptoms include lethargy, somnolence, hypotonia, ataxia, slurred speech, etc.
- Respiratory depression results from a lack of CNS respiratory drive coupled with hypotonia of the airway muscle can lead to life-threatening hypoxia.
- If BZD overdose is suspected, supportive measures should be taken, including airway management and fluid replenishment for hypotension. It is also important to rule out other causes for overdose that present similarly (i.e., opioids) and treat concomitant overdose accordingly (i.e., naloxone).
- Flumazenil, a competitive GABA receptor antagonist, is indicated in limited situations for use in BZD-naïve patients with accidental ingestion patients or perioperative oversedation. However, flumazenil use carries a high risk of seizures and arrhythmias and is contraindicated in patients who chronically use BZDs, history of seizure disorder, or at risk for seizures.9
- For overdose in adults, the starting dose of flumazenil is 0.2 mg IV. Further doses of 0.3-0.5 mg IV can be given if consciousness is not restored, up to a maximum total dose of 3 mg. After 5 mg without response, the primary sedative agent is not a BZD. For reoccurrence, doses can be given at 20-minute intervals, no more than 1 mg/dose or 3 mg/hr.
References
- Sanabria E, Cuenca RE, Esteso ME, et al. Benzodiazepines: Their use either as essential medicines or as toxics substances. Toxics. 2021;9(2):25. PubMed
- Griffin CE, 3rd, Kaye Am, Bueno FR, et al., Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013. 13(2): 214-23. PubMed
- McIntosh B, Clark M, Spry C. Benzodiazepines in older adults: A review of clinical effectiveness, cost-effectiveness, and guidelines. 2011: Ottawa (ON). PubMed
- Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. 2017;376(12):1147-57. PubMed
- Intravenous Anesthetics. In: Butterworth IV JF, Mackey DC, Wasnick JD. eds. Morgan & Mikhail’s Clinical Anesthesiology, 7e. McGraw-Hill Education; 2022.
- Edinoff AN, Nix CA, Hollier J, et al., Benzodiazepines: Uses, dangers, and clinical Considerations. Neurol Int. 2021; 13(4): 594-607. PubMed
- Cochen De Cock V., Sleepwalking. Curr Treat Options Neurol. 2016;18(2): 6. PubMed
- Mancuso CE, Tanzi MG, Gabay M/ Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. 2004; 24(9): 1177-85. PubMed
- Kang M., Galuska MA, and Ghassemzadeh S. Benzodiazepine toxicity. In: StatPearls. 2024: Assessed 2/2/2024. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.