Copy link
Caudal Anesthesia in Children
Last updated: 03/14/2023
Key Points
- Neonates and infants have lower termination of the spinal cord than adolescents and adults, which necessitates a caudal approach to the epidural space for safe neuraxial blockade. Caudal anesthesia is safe, widely performed, and has a diverse range of intra and postoperative utility.
- Caudal anesthesia, using either a single-shot injection or catheter, can be used as the sole anesthetic for surgeries below the umbilicus but is mostly used as an adjunct to general anesthesia and for postoperative analgesia.
- Proper knowledge of anatomy, contraindications, aseptic technique, procedural steps, local anesthetic dosing, and test dosing are essential for providers to recognize and avoid complications of caudal blockade.
Introduction
- Neuraxial procedures for anesthesia and analgesia in infants are widely used, and the already diverse indications are continually evolving. Infiltration of local anesthetic into the caudal epidural space is one of the most common regional techniques used for small children.
- Caudal anesthesia can be reliably employed for numerous surgeries below the umbilicus, such as hernia repair, urologic procedures, and orthopedic surgeries of the hip and lower extremities. Caudal block can be used to provide surgical anesthesia when the provider wants to avoid volatile anesthetic and/or minimize opioid use, such as with preterm neonates (to reduce perioperative apnea), patients with a history of malignant hyperthermia, or those with severe pulmonary pathology. More often, caudal anesthesia is used as an adjunct to general anesthesia and results in a lower volatile anesthetic requirement. Placement of a catheter can provide pain control in the postoperative period.1
- All neuraxial procedures incur risk to the patient, and long-term, permanent complications have been published. Nevertheless, caudal anesthesia is a safe and reliable method for achieving low-thoracic and lumbosacral level analgesia for children undergoing surgery.2
Contraindications
- General contraindications to neuraxial procedures include
- patient or guardian refusal;
- infection overlying the lumbar and sacral area (infected pilonidal cysts);
- increased intracranial pressure;
- use of blood thinners or a coagulopathy that can increase the risk of an epidural hematoma; and
- open neural tube defects, unrepaired tethered cord, complex spina bifida, presence of neurological dysfunction (i.e., bowel/bladder incontinence).3
- Simple sacral dimple versus complex sacral stigmata
- Simple sacral dimple does not preclude safe epidural placement. It is important for providers to distinguish a dimple from complex stigmata using preprocedural MRI or
ultrasound. Complex sacral abnormalities or tethered cord increase the risk of cord trauma or compression from injectate volume.3,4
- Simple sacral dimple does not preclude safe epidural placement. It is important for providers to distinguish a dimple from complex stigmata using preprocedural MRI or
- Prior instrumentation and placement of hardware in the lumbosacral region, severe scoliosis, and sacrococcygeal abnormalities can make safe placement challenging, and individual risks should be weighed against the benefits. Successful epidural placement has been safely performed in patients following detethering, and after repair of myelomeningocele.4
- There is no specific age or size cutoff, but many providers avoid placement after the child begins to walk, especially for same-day surgeries.
Anatomy of the Caudal Space and Relationship to the Dural Sac
Anatomic Considerations in Neonates and Infants
- The spinal cord in an infant typically ends at the L3/L4 vertebral level, which means lumbar epidural placement (classically done in parturients) for neonates and infants carries an increased risk of cord damage from the needle. Caudal infiltration of local anesthetic is a safer alternative. The infant’s dural sac also ends lower than adults: at S3/4 in small children versus the adult S1/2 level. This means unintentional dural puncture is possible during a caudal block.1
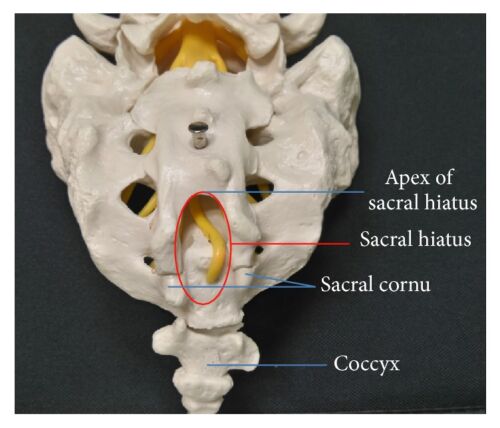
Figure 1. Anatomy of caudal epidural space. SH = sacral hiatus. Source: Kao SC, Lin CS.
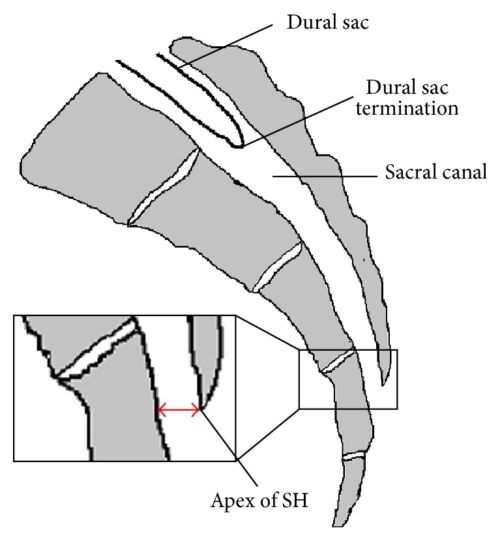
Figure 2. Caudal epidural block: An updated review of anatomy and techniques. Biomed Res Int. 2017; 2017:9217145. CC BY SA 3.0. Link
- The target for a caudal block is the caudal epidural space, which contains the filum terminale, cauda equina, and sacrococcygeal spinal nerves. The canal is entered through the sacral hiatus, which becomes less prominent as children age. The hiatus is bordered laterally by two bony sacral cornua, which are usually palpable just superior to the gluteal cleft. The skin, subcutaneous tissue, and finally, the sacrococcygeal ligament are punctured to enter the canal.
Surface Anatomy
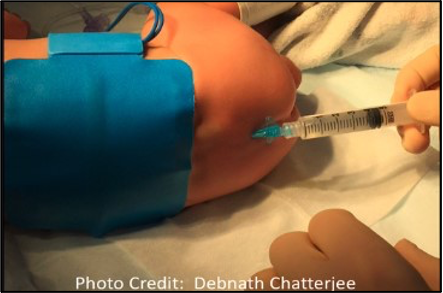
Figure 3. Positioning, with surface landmarks visible, for approaching the caudal space.
- In the lateral decubitus position, with hips and knees flexed, an equilateral triangle is formed between the two posterior superior iliac spines, with the coccyx as the apex. The cornua can be appreciated as small bony prominences, just cephalad to the gluteal cleft. The hiatus exists as a tunnel running at a shallow angle to the patient, with the cornua serving as lateral borders. Entry into the dural sac also occurs superficially in young patients, as shallow as 1-1.5 cm in patients under three years of age.1
Procedure
Preparation
- Successful placement of the block can be accomplished using light sedation or none at all. Most providers agree that the risk of damage from a moving, uncooperative (and typically nonverbal) patient is high, and thus general anesthesia is usually induced prior to placing the patient in the lateral decubitus position, with the hips and knees flexed. A sterile field is created, and sterile gloves are worn.
- For a single-shot caudal, a 23-gauge butterfly needle or 22-gauge intravenous (IV) catheter is commonly employed. Indwelling catheter placement requires a needle gauge larger than the infusion catheter, and an 18- or 22-gauge Tuohy, caudal needle, or 18-gauge IV catheter can be used, with the infusion catheter threaded into the lumen of the IV catheter used for puncture.
Identification of Landmarks
- The provider should identify the approximate location of the sacral hiatus. See above section entitled “Surface Anatomy.”
Needle and Catheter Insertion
- The sacral hiatus is palpated using the nondominant hand, and the needle is inserted at a 45-degree angle through the skin, as the provider attempts to appreciate the “pop” of the sacrococcygeal ligament. Once through the ligament, the angle is decreased, and if using a catheter, the needle is further advanced 2-3 millimeters until the catheter threads off easily. The infusion catheter can be placed through the entry needle/catheter and secured to the skin. If performing a single shot, constant palpation above the entry site can detect subcutaneous infiltration.
- The use of continuous electrocardiography, pulse oximetry, and cycling of blood-pressure measurement should be employed during block placement.
- Loss of anal sphincter tone is highly sensitive for detecting successful block. An audible “swoosh” sound can also be auscultated by a stethoscope over the sacrum during infiltration.5
Ultrasound-Guided Caudal Block
- Ultrasound guidance is emerging as an effective aid for performing the caudal block.
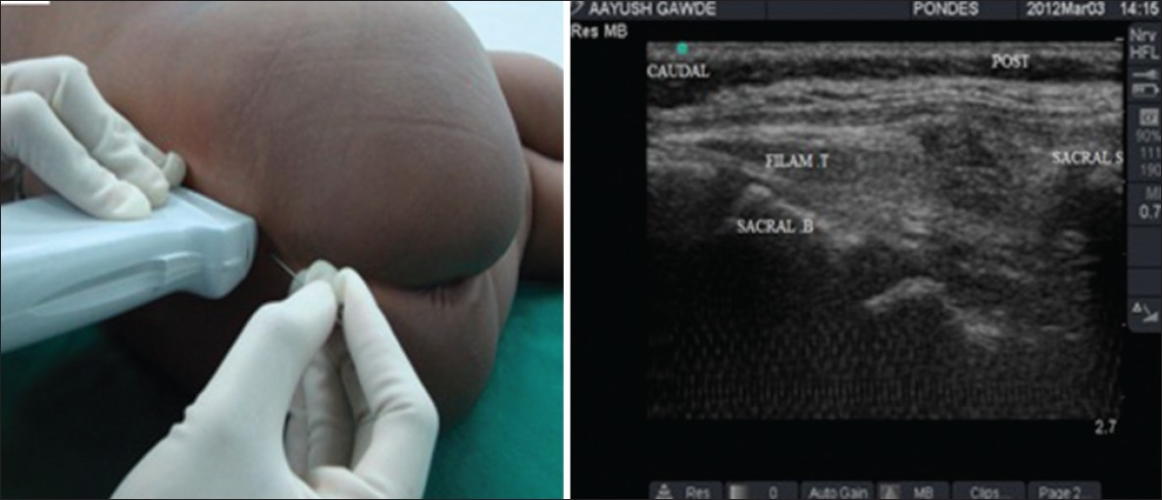
Figure 4. Ultrasound-guided caudal block. Source: Ponde VC. Indian J Anaesth. 2012;56(5):470-8. PubMed . CC BY NC SA 3.0
- Typically, the cornua and sacrococcygeal membrane are visualized in short-axis (Figure 5) before rotating the probe to the long-axis (Figure 6) to guide the needle and catheter into the sacral hiatus. Ultrasound has been shown to decrease the rate of block failure, but not the rate of complications, and test dose must still be administered.
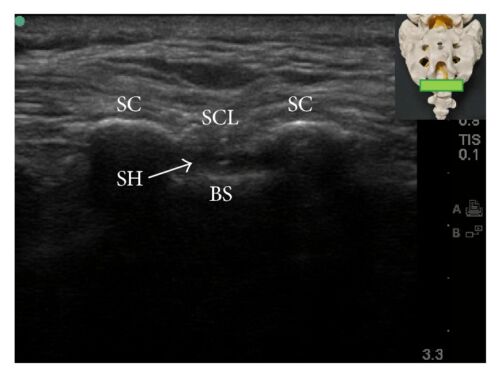
Figure 5. Short and long axis view of the sacral hiatus during caudal block. BS = base of sacrum; SC = sacral cornua; SCL = sacrococcygeal ligament; SH = sacral hiatus. Source: Kao SC, Lin CS.
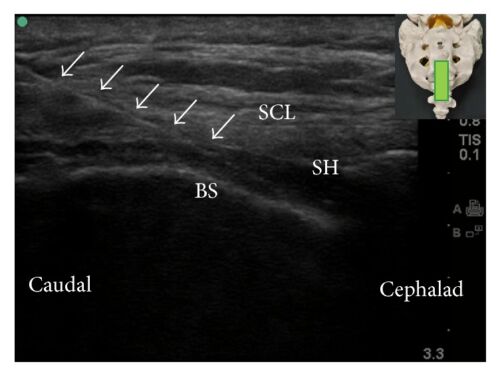
Figure 6. Caudal epidural block: An updated review of anatomy and techniques. Biomed Res Int. 2017; 2017:9217145. CC BY SA 3.0. Link
Dosing
Test Dosing
- Aspiration should be performed to detect inadvertent subarachnoid or intravascular placement. Negative aspiration does not have perfect sensitivity for intravascular or intraosseous placement. Administration of a test dose of local anesthetic is the essential next step in the detection of incorrect placement.
- A typical test dose volume is 0.1 mL/kg of lidocaine 1.5% with epinephrine 1:200,000, and the patient is monitored for 60-90 seconds. Hemodynamic changes suggestive of systemic injection include T-wave changes (especially in neonates), heart rate increase by more than 10 beats per minute and an increase in systolic blood pressure over 10 mmHg. Intrathecal injection is difficult to detect in the awake or anesthetized infant, as children display less hypotension than adults from resulting sympathectomy.
- There is concern from recent studies for spinal ischemia following large amounts of epinephrine administration with the local anesthetic. For this reason, many providers limit epinephrine to the test dose.6
Local Anesthetic Choice and Volume
- The duration of blockade depends on the choice of local anesthetic, dose/concentration of local anesthetic, and the use of adjuvants. Ropivacaine and bupivacaine are the most common local anesthetics used in a caudal block.
- The drug dose for coverage up to a certain dermatomal level depends on several factors, including the volume (not the concentration) of the local anesthetic and the capacitance of the epidural space, which varies inversely with age. According to the classic Armitage formula, which was based on clinical experience, a volume of 0.5 mL/kg local anesthetic provides lumbosacral coverage, 1 mL/kg provides thoracolumbar coverage, and 1.25 mL/kg provides coverage up to the mid-thoracic level.7
- However, the exact volume needed for reaching a particular dermatomal height is difficult to determine. Magnetic resonance imaging studies have shown that the volume of the epidural space is not uniform and is significantly higher at the lumbar level [1.18 mL/segment (95% CI 0.94-1.43)] compared with the caudal [0.85 mL/segment (95% CI 0.56-1.18)] and thoracic levels [0.60 mL/segment (95% CI 0.38-0.75)].8
- Additionally, ultrasound studies to determine anatomically where the local anesthetic reaches are affected by a phenomenon known as cerebrospinal fluid (CSF) rebound.9 When the local anesthetic is initially injected into the epidural space, it displaces some of the CSF into the intrathecal sac cephalad. However, over the next 15 minutes or so, the CSF “rebounds,” causing the dural sac to re-expand, which in turn forces the local anesthetic in the epidural space cephalad, resulting in a higher level.
- Another approach is to use Takasaki’s formula of 0.056 mL/kg per dermatome to be blocked.10 Therefore, T10 coverage in a 10 kg child will require 0.056 x 10 kg x 12 dermatomes or 6.72 mL of local anesthetic.
Toxicity and Complications
Intravascular or Subarachnoid Injection
- Aspiration for blood or cerebrospinal fluid is performed immediately after entry into the caudal space. Next, the administration of a test dose containing epinephrine can detect systemic injection of local anesthetic. See above section “Test Dosing.”
- As with any regional or neuraxial block, the administration of intravascular local anesthetic results in local anesthetic systemic toxicity (LAST). The neurologic effects are impossible to detect in the anesthetized patient, so circulatory collapse may be the first indication. Care must be taken to ensure proper placement. Communication with the surgeon can ensure that over-administration of local anesthetic at the surgical site does not occur following a regional procedure.
Complications
- Large, multicenter, retrospective reviews have demonstrated the safety of caudal anesthesia, and long-term complications are rare. During placement, care must be taken to avoid dural puncture, as children over the age of five are still susceptible to postdural puncture headaches. Damage to the spinal cord has been reported. Perforation of the rectum can result in catastrophic meningitis if the needle is then passed into the epidural space.1,2
- The most common complications are block failure, headache, low-back pain, and transient neurological symptoms.2
References
- Lang RS, Hall-Burton D, Praslick A, et al. Regional anesthesia. In: Davis P and Cladis FP (eds). Smith’s Anesthesia for Infants and Children, 10th edition. Philadelphia, PA;Elsevier; 2022: 533-35.
- Suresh S, Long J, Birmingham P, De Oliviera G. Are caudal blocks for pain control safe in children? An analysis of 18,650 caudal blocks from the pediatric regional anesthesia network (PRAN) database. Anesth Analg. 2015; 120:151-6. PubMed
- McLone DG, Dias MS. The Chiari II malformation: cause and impact. Childs Nerv Syst. 2003.19:540-50. PubMed
- Masaracchia MM, Sunder RA, Polaner DM. Error traps in pediatric regional anesthesia. Paediatr Anaesth. 2021; 31(11): 1161-89. PubMed
- Verghese S, Mostello L, Patel R, et al. Testing anal sphincter tone predicts the effectiveness of caudal analgesia in children. Anesth Analg. 2002; 94:1161–4. PubMed
- Tobias J. Caudal Epidural Block: A review of test dosing and recognition of systemic injection in children. Anesth Analg. 2001; 93:1156–61. PubMed
- Armitage EN. Is there a place for regional anesthesia in pediatrics? Yes! Act Anaesthesiol Belg. 1988:39:151-6. PubMed
- Forestier J, Castillo P, Finnbogason T, et al. Volumes of the spinal canal and caudal space in children zero to three years of age assessed by magnetic resonance imaging: implications for volume dosage of caudal blockade. Br J Anaesth. 2017; 119(5): 972-8. PubMed
- Brenner L, Marhofer P, Kettner SC, et al. Ultrasound assessment of cranial spread during caudal blockade in children: the effect of different volumes of local anesthetics. Br J Anaesth. 2011;107(2): 229-35. PubMed
- Takasaki M, Dohi S, Kawabata Y, et al. Dosage of lidocaine for caudal anesthesia in infants and children. Anesthesiology.1977; 47(6): 527-9. PubMed
Other References
- Ambardekar A. Caudal Epidural Block. OpenAnesthesia. Published March 1, 2017. Accessed March 2, 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.