Copy link
Acute Cocaine Intoxication
Last updated: 05/22/2023
Key Points
- Cocaine, a naturally occurring sympathomimetic alkaloid, exerts its effects by blocking presynaptic noradrenergic, serotonergic, and dopaminergic transporters and prevents the reuptake of each respective neurotransmitter resulting in prolonged effects on the postsynaptic receptors. Cocaine also exerts local anesthetic effects by blocking sodium channels.
- Acute cocaine intoxication affects several organ systems and presents with a wide range of symptoms.
- Selective beta-blockers should be avoided in the acutely intoxicated patient secondary to the risk of unopposed alpha-mediated coronary and peripheral vasoconstriction.
- Catecholamine depletion in patients abusing cocaine renders these patients unresponsive to indirectly acting sympathomimetics such as ephedrine.
Introduction
- Cocaine is a naturally occurring sympathomimetic alkaloid, isolated from the plant Erythroxylon coca, that indigenous Andean tribes originally consumed to relieve fatigue, cold, hunger, and altitude sickness.1
- Though first extracted in 1855, cocaine was medicalized in 1884 after Karl Koller administered cocaine as a local anesthetic to the eye of a patient for an ophthalmologic procedure. The revelation of this application opened the door to the field of regional anesthesia.
- Cocaine is one of the most common recreational drugs of abuse with prevalence in up to 1-2% of the adult population. In the United States, cocaine is considered a Schedule II drug with high abuse potential. Oregon is the only state where the possession of cocaine is decriminalized for amounts under 2 grams.1
Pharmacokinetics
- Cocaine is commonly found in two forms.1
- Cocaine hydrochloride (also known as “coke,” “blow,” or “snow”) is a fine white crystalline powder, which is soluble in water and is consumed intranasally (“snorting,” or “sniffing”), orally, or intravenously.
- Cocaine free-base (also known as “crack cocaine”) that is consumed via inhalation (smoking). The name is derived from the cracking noise made when the rock-like cocaine is heated.
- Cocaine is well absorbed following oral, nasal, inhalational, gastrointestinal, rectal, and vaginal administration. Its bioavailability is 90% via inhalation and 80% after intranasal route.
- The onset of action varies (3-5 seconds after inhalation, 10-60 seconds after intravenous, and 5 minutes after intranasal use (delayed secondary to topical vasoconstriction).2
- The duration of effects is longest with intranasal route (60-90 minutes) and shortest with inhalation (5-15 minutes).2
- Cocaine is rapidly metabolized by plasma and liver cholinesterases, and the major metabolites are benzoylecgonine (BE), ecgonine methyl ester (EME), and norcaine. When combined with ethanol, a unique compound called cocaethylene is formed, which has a longer duration of action and is more potent than cocaine itself.2
- The half-life of cocaine is 0.5-1.5 hours. BE has a serum half-life of 5-8 hours, and EME has a serum half-life of 3.5-6 hours.
Mechanism of Action
Cocaine has multiple effects at the neurotransmitter synapse (Figure 1).
- At lower concentrations, cocaine binds and blocks monoamine reuptake transporters
- It indirectly stimulates the alpha-1, alpha-2, beta-1, and beta-2 adrenergic receptors by blocking noradrenergic transporters (NAT) and thus increasing levels of norepinephrine. This sympathomimetic activity results in its cardiovascular effects.
- It directly blocks dopamine transporters (DAT) and 5-HT serotonin transporters (SERT) to increase dopamine and serotonin concentrations, resulting in its euphoric and addictive properties.1,2
- At higher concentrations, cocaine blocks the voltage-gated Na+ channels, thereby inhibiting action potentials in neurons and cardiac myocytes. This explains the local anesthetic effects of cocaine.1,2
- Additional actions are seen with its effects on muscarinic acetylcholine, N-methyl D-aspartate, sigma, and kappa-opioid receptors.
- Cocaine also increases the concentrations of excitatory neurotransmitters glutamate and aspartate in the brain.
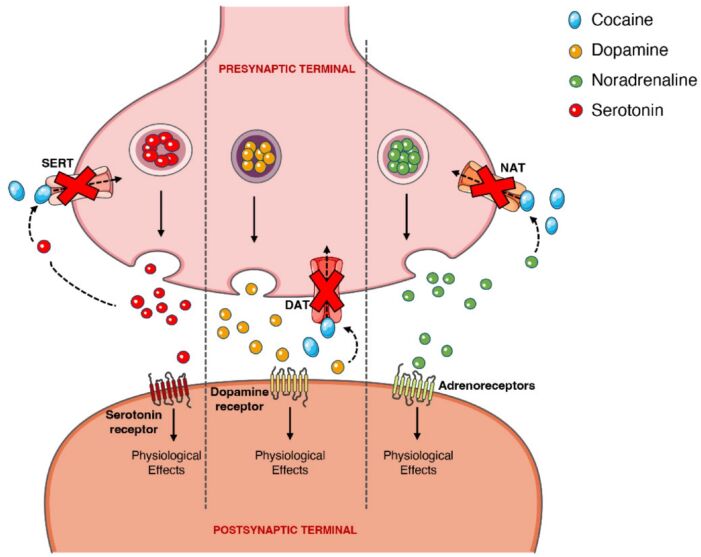
Figure 1. Effects of cocaine at the noradrenergic, serotonergic, and dopaminergic receptors. Blockade of the noradrenergic, serotonergic, and dopaminergic presynaptic transporters prevents reuptake of each respective neurotransmitter resulting in prolonged effects on the postsynaptic receptors. Source: Bravo RR, et al. Cocaine: An updated overview on chemistry, detection, biokinetics, and pharmacotoxicological aspects including abuse pattern. Toxins. 2022;14(4):278. CC BY. Link.
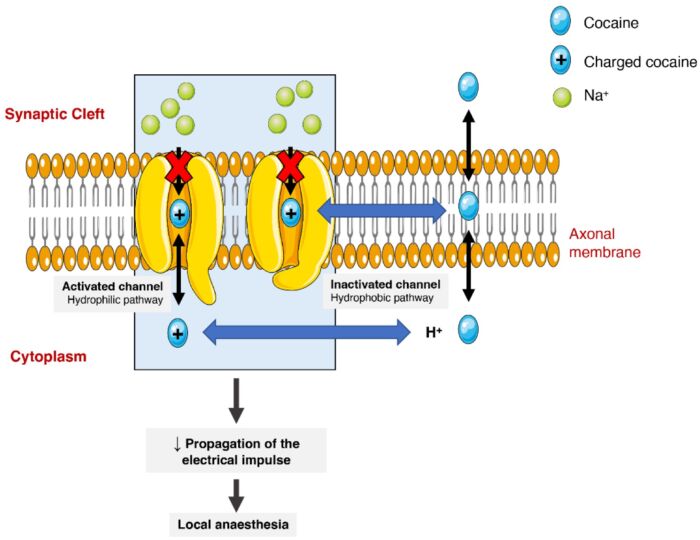
Figure 2. Effect of cocaine on voltage-gated sodium channels. Cocaine binding to the sodium channel in two fashions (hydrophilic and hydrophobic) and thereby prevents Na+ ions from crossing the channel to inhibit the propagation of action potentials. Source: Bravo RR, et al. Cocaine: An updated overview on chemistry, detection, biokinetics, and pharmacotoxicological aspects including abuse pattern. Toxins. 2022;14(4):278. CC BY. Link.
Effects on Organ Systems1-5
Central Nervous System (CNS)
- Increased dopamine and serotonin results in psychomotor effects. Low levels cause euphoria, anxiety, restlessness while higher levels induce agitation, hallucinations, and psychosis. These patients are at an increased risk for seizures, coma, intracranial hemorrhage, and strokes.
HEENT
- Nasal septal breakdown/perforation, mydriasis, bruxism
Cardiovascular
- Increased norepinephrine and epinephrine uptake results in arterial vasoconstriction. Tachycardia, hypertension, and increased myocardial O2 demand increase the risk for myocardial infarction.
- At higher doses, cocaine’s effect of sodium channel blockade results in negative inotropic effects and widening of the QRS complex/QT interval, which can lead to acute nonischemic heart failure as well as ventricular ectopy or dysrhythmias.
- There have been reports of increased arterial and venous thrombus events due to ADP and plasminogen activator inhibitor mediated pathway promoting platelet activation and thrombus formation.
- Chronic use can cause accelerated atherosclerosis and ventricular hypertrophy.
Pulmonary
- Most pulmonary complications are associated with inhaling cocaine. Acutely, patients can present with shortness of breath, acute pulmonary toxicity, barotrauma, pneumothorax, pneumomediastinum, eosinophilic pneumonia, and respiratory failure.
- Chronic use can lead to interstitial fibrosis, alveolar hemorrhage, and pulmonary hypertension.
Gastrointestinal
- Complications arise from vasoconstriction at local sites such as gastric perforation and local gut ischemia.
Renal
- Acute kidney injury can be seen along with interstitial nephritis, glomerulonephritis, and renal infarction.
Endocrine
- Increased cortisol secretion
Musculoskeletal
- Tremors are often associated with the psychomotor agitation. There is also an increased risk for rhabdomyolysis.
Cutaneous
- Hyperthermia and pruritis can be commonly seen in the initial presentation.
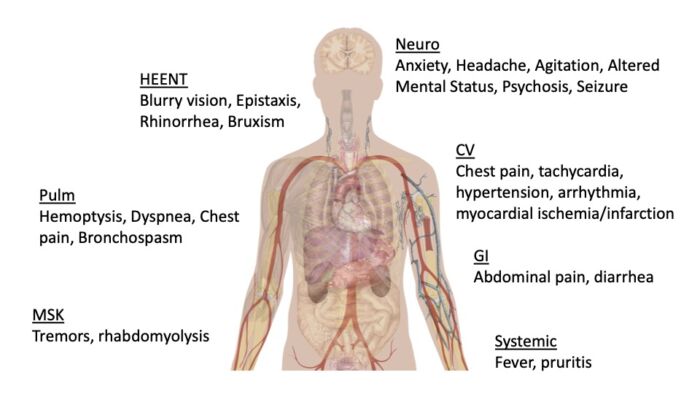
Figure 3. Systemic effects of acute cocaine intoxication. Adapted from Mikael Häggström, CC0 via Wikimedia Commons. Link.
Management of Acute Cocaine Intoxication1-5
- CNS/psychomotor agitation: Benzodiazepines should be used as the first line agent for the treatment of agitation. Lorazepam, midazolam, and diazepam via intravenous or intramuscular routes are common choices. Haloperidol should be avoided as it can lower the seizure threshold. Seizures are usually self-limited but may need treatment with benzodiazepines.
- Hypertension: Benzodiazepines are used to decrease CNS-mediated catecholamine release. Alpha blockade can be targeted with phentolamine or vasodilation WITHOUT beta blockade using nitroglycerin or nitroprusside. Beta blockers should be avoided to treat cocaine-induced hypertension and tachycardia due to concerns for unopposed alpha stimulation. In some studies, the mixed alpha and beta blocker, labetalol, has been shown to be safe to treat cocaine-induced hypertension and tachycardia; however, these studies are weak, and more information is needed before recommending its routine use.
- Hypotension: Hypotension can be secondary to sodium channel blockade, dysrhythmia, or myocardial infarction. Intravenous fluids should be administered, with controlled titration of a direct-acting vasopressor being the next line agent if fluids are not effective.
- Acute coronary syndrome (ACS)/ myocardial infarction (MI): ACS symptoms can occur from several hours to weeks after exposure due to cocaine. Patients should receive aspirin if there is no concern for aortic dissection and symptoms are thought to be secondary to ischemia. Nitroglycerin and benzodiazepine are the primary treatment for ongoing chest pain.
- Ventricular tachydysrhythmia: If there is evidence of QRS widening, sodium bicarbonate or amiodarone administration should be considered. Intravenous lipid emulsion has been used to treat with success in case reports. Treatments with calcium channel blockers and adrenergic antagonists have also been successful.
- Pulmonary toxicity: Initial treatment includes supplemental oxygen and bronchodilator therapy if wheezing is present. If there is concomitant acute respiratory distress syndrome (ARDS), it should be managed per ARDS protocol. If there is acute eosinophilic pneumonia, systemic glucocorticoids should be considered (otherwise steroids are not indicated). Consider intubation if the patient has a risk for respiratory failure.
- Gastrointestinal: If there are concerns about enteral absorption, the source should be removed through nasal irrigation and activated charcoal. If there are concerns for intrabdominal cocaine via body packing, whole bowel irrigation or surgical removal should be considered.
- Hyperthermia: External cooling measures, including ice pack, cooled intravenous fluids, and gastric lavage should be initiated.
Anesthetic Considerations5,6
- Clear consensus guidelines are lacking; although, there is general agreement amongst anesthesiologists to not proceed with an elective procedure if a patient has acute cocaine intoxication. Additionally, many institutions will cancel a procedure for a positive urine drug screen (UDS).
- For asymptomatic patients with a positive cocaine on UDS, studies have shown that they have similar cardiovascular stability and surgical outcomes while undergoing general anesthesia compared to drug-free control groups.
Preoperative Evaluation
- A detailed history and physical exam should be performed to assess if the patient is acutely intoxicated with symptoms. It may be useful to consider obtaining an ECG to assess for QT prolongation and evidence of myocardial injury.
Intraoperative Considerations
- Despite commonly accepted teaching that acute cocaine intoxication increases the minimum alveolar concentration of volatile anesthetics, more recent studies have shown that there is no difference in anesthetic requirement. Chronic use, however, may still increase overall anesthetic requirements.
- If rapid sequence induction is required for emergent intubation, administration of succinylcholine should be avoided as coadministration can prolong both the effects of cocaine and succinylcholine since they are both metabolized by pseudocholinesterase.
- Hemodynamics should be closely monitored during acute intoxication due to initially increased and then depleted circulating catecholamines. Phenylephrine is the preferred agent for blood pressure support as patients may not have the same response to ephedrine.
- Medications such as ketamine that could potentially worsen the cardiovascular toxicity of cocaine or cause myocardial depression due to depleted catecholamines should be avoided.
Postoperative Care
- Close hemodynamic monitoring should be continued postoperatively.
- Symptoms of cocaine withdrawal include agitation, anxiety, depression, insomnia, and myalgias.
Cocaine Abuse During Pregnancy
- Cocaine abuse during pregnancy is associated with several obstetric complications, including preterm labor, premature rupture of membranes, spontaneous abortion, abruptio placentae, stillbirth, maternal hypertension, etc.
- In addition to all the cardiovascular, neurological, and respiratory complications listed previously, cocaine use may cause thrombocytopenia and prolong bleeding times. The patient’s platelet count should be checked before neuraxial anesthesia.
- Phenylephrine is preferred over ephedrine for the treatment of hypotension.
- Metabolism of ester local anesthetics may be delayed because they are also metabolized by plasma cholinesterases.
References
- Bravo RR, Faria AC, Brito-da-Costa AM, et al. Cocaine: An updated overview on chemistry, detection, biokinetics, and pharmacotoxicological aspects including abuse Pattern. Toxins. 2022;14(4):278. PubMed
- Zimmerman JL. Cocaine Intoxication. Crit Care Clin. 2012;28(4):517-26. PubMed
- Richards JR, Le JK. Cocaine toxicity. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing; 2023. Accessed February 20, 2023. Link
- Nelson LS, Odujebe O. Cocaine: acute intoxication. In: Post T, ed. UpToDate; 2023. Accessed 4/15/23. Link
- Butterworth J, Mackey D, Wasnick J. Local Anesthetics, Anesthesia for Patients with Neurological & Psychiatric Diseases. Morgan and Mikhail's Clinical Anesthesiology. 7th edition. New York; McGraw-Hill; 2022: 268-271, 633-634.
- Moon TS, Gonzales MX, Sun JJ et al. Recent cocaine use and the incidence of hemodynamic events during general anesthesia: A retrospective cohort study. Journal of Clinical Anesthesia. 2019; 55: 146-150. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.