Copy link
Apnea of Prematurity and Postoperative Apnea
Last updated: 05/23/2023
Key Points
- Apnea of prematurity is considered a developmental disorder and is diagnosed in infants who have apneic episodes lasting 20 seconds or more, or if shorter apneic episodes are associated with bradycardia or desaturation.
- The incidence of apnea of prematurity is inversely proportional to gestational age.
- Most apneic episodes in premature infants are mixed events, when obstructed airflow results in a central apneic pause or vice versa.2
- All infants less than 28-weeks gestational age generally have apnea of prematurity-related symptoms, decreasing to only about 20% at 34 weeks, and less than 10% beyond 34-weeks gestation.
- Infants with low gestational age, low postconceptual age, apnea of prematurity, multiple congenital anomalies, anemia, and chronic lung disease are at higher risk of postoperative apnea.
Age Terminology
- Using consistent definitions to describe the length of gestation and age of neonates is important (Table 1 and Figure 1).1
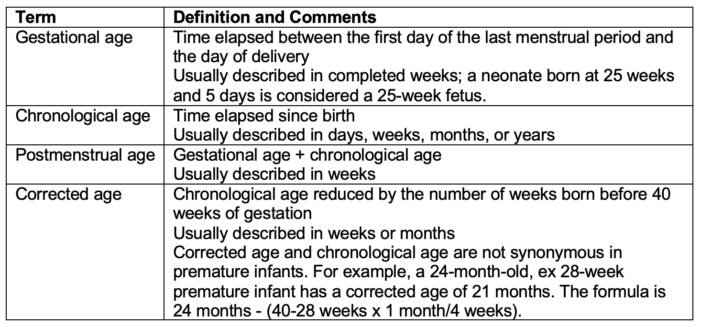
Table 1. Age terminology
- Although primarily used in classic anesthesiology literature; the terms postconceptual age, conceptional age, or postconceptional age are no longer standard terminology in perinatology literature.1,9
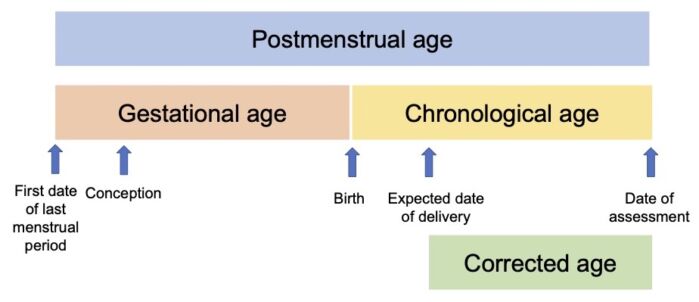
Figure 1. Age terminology in the perinatal period. Adapted from Engle WA, et al. Age terminology during the perinatal period. Pediatrics. 2004;114(5):1362-4.1
Introduction
- A clinical diagnosis of apnea of prematurity is made if a premature infant has a cessation of breathing for at least 20 seconds or a shorter pause associated with bradycardia (less than 100 beats per minute) or desaturation.2-4
- Apnea may be classified as central (cessation of breathing effort), obstructive (airflow obstruction usually at the pharyngeal level), or mixed.
- Most apneic episodes in premature infants are mixed events, when obstructed airflow results in a central apneic pause or vice versa.2
- Etiology is likely from immature neurons in the medullary respiratory control centers as well as immaturity of the peripheral chemoreceptor triggering respiration.
- Hypoxemia leads to bradycardia because newborns have a fully matured parasympathetic system and an immature sympathetic system. Premature infants are more prone to hypoxemia given their immature control of breathing.
- The incidence of apnea of prematurity is inversely proportional to gestational age.2-4
- Virtually all infants born at less than 28 weeks gestation develop apnea of prematurity, and the incidence decreases to 85% of infants born at 30 weeks and 20% of infants born at 34 weeks gestation.2
- Therefore, all premature infants born at less than 35-weeks gestational age require monitoring for apnea.
- By postconceptual age of 52 weeks, these apneic episodes typically resolve.
Control of Breathing in Premature Infants
- The pathogenesis of apnea of prematurity remains poorly understood but is thought to be associated with immature control of breathing.2-4,10
- In addition to aberrant activity of central and peripheral chemoreceptors, preterm infants also have poor neuromuscular control of upper airway patency.
- Immature brainstem control leads to periodic breathing which refers to oscillations between brief episodes of tachypnea causing hypocarbia leading to apnea in a cyclic pattern causing intermittent hypoxia.10
- Several factors that have been implicated in the pathogenesis of apnea of prematurity.2
- Central mechanisms:
- Decreased central chemosensitivity
- Hypoxic ventilatory depression
- Upregulated inhibitory neurotransmitters
- Delayed central nervous system development
- Peripheral reflex pathways:
- Immature carotid body reactivity
- Laryngeal chemoreflex
- Excessive bradycardic response (parasympathetic mediated)
- Central mechanisms:
Risk Factors for Apnea of Prematurity
- Risk factors include:2-4,9
- Anemia
- Glucose or electrolyte imbalance
- Presence of a patent ductus arteriosus with a large shunt
- Medications including opioid analgesics and magnesium sulfate
- Gastroesophageal reflux
- Elevated body temperature
Treatment
- Acute apneic episodes should initially be treated with simple stimulation and airway maneuvers; manual bag-mask ventilation is reserved for prolonged or profound episodes.10
- The main prophylactic pharmacologic treatment for apnea of prematurity and postoperative apnea remains methylxanthines.2-4
- Caffeine and theophylline can be used, but caffeine is preferred given its longer half-life and higher therapeutic index.
- Standard regimens include a loading dose of 20 mg/kg followed by a 5-10 mg/kg/day maintenance dose.2 Higher dosing regimens have been shown to be more effective but may be associated with increased side effects.
- Theophylline requires serum drug level monitoring.
- Methylxanthines block inhibitory adenosine A1 receptors, which lead to excitation of respiratory neural output, and block excitatory adenosine A2A receptors found on gamma-aminobutyric acidergic neurons.
- Adverse effects of xanthine therapy include tachycardia, emesis, and jitteriness.
- Several studies have shown that prone positioning reduces apnea of prematurity by improving thoracoabdominal synchrony and stabilizing the chest wall without affecting the breathing pattern or SpO2. However, if the patient is receiving pharmacological treatment, it does not provide any further improvement in apnea of prematurity.4
- Physical adjuncts such as nasal continuous positive airway pressure (NCPAP) at pressure 4-6 cm H2O are effective at reducing the severity and frequency of apnea in preterm infants especially when used in conjunction with methylxanthines.2-4,10
- Blood transfusions have been used as a treatment.
- Increase in oxygen-carrying capacity, total content of oxygen in the blood, and tissue oxygenation are thought to increase the respiratory drive.
- Retrospective and prospective studies that have sought to prove efficacy are conflicting and fail to show that blood transfusions result in any long-term improvements in apnea.
- Despite the lack of definitive studies, some speculate that treating gastroesophageal reflux (GER) helps to decrease the incidence and severity of apnea of prematurity because the hyperreactive laryngeal chemoreflex has been shown to precipitate apneas when stimulated.2-4
Postoperative Apnea
- Not surprisingly, preterm infants less than 44 weeks postconception age (PCA) are at a greater risk for apnea after sedation or general anesthesia than infants more than 44 weeks PCA.
- Combined analysis of data from 8 prospective studies of premature infants undergoing herniorrhaphy by Cote et al. showed the following.5
- The incidence of apnea following inguinal hernia repair did not fall below 5% until PCA of 48 weeks in premature infants born at 35-weeks GA or until a PCA of 50 weeks for infants born earlier than 32 weeks GA.
- The incidence of apnea fell below 1% once the PCA reached 54 weeks in infants born at 35 weeks GA or PCA of 56 weeks in infants born at 32 weeks GA.
- The incidence of postoperative apnea was inversely proportional to both gestational age and PCA.
- Anemia is a significant risk factor for postoperative apnea, particularly in infants of more than 44 weeks GA.
- Small-for-GA infants are protected from apnea compared with appropriate- or large-for-GA infants.
- The following are the recommendations for the discharge of preterm and term infants based on their PCA.6,7
- Elective or nonurgent cases for infants (both preterm and term) less than 50 weeks PCA should be delayed, if possible due to the risk of postoperative apnea.
- Premature infants younger than 55 weeks PCA, those who are anemic (hematocrit <30%), and those with apnea should be admitted for monitoring.7
- Former preterm infants presenting at 55-60 weeks PCA and who are not anemic or experiencing apnea can be observed for an extended period in the postanesthesia care unit (PACU) and if stable, later discharged.7
- The incidence of apnea and bradycardia is highest within the first 4-6 hours after surgery, but these events are reported for up to the first 12 hours. Therefore, the infant should be monitored for at least 12 hours after surgery.
- Postoperative apnea in premature infants older than 60 weeks PCA has not been reported, therefore many institutions opt to take the conservative approach and write policies to reflect the practice of admitting any premature infant under 60 weeks PCA.7
- Full-term infants under 44 weeks PCA should be admitted overnight for monitoring.6
- Full-term infants with a history of apnea and bradycardia or those with a sibling with sudden death syndrome should be observed for an extended period in the PACU or admitted overnight for monitoring.7
- Full-term infants older than 44 weeks PCA can be discharged home after an extended PACU observation only if they have a completely uneventful recovery with adequate pain control and adequate fluid intake.6
Regional Anesthesia and Postoperative Apnea
- Regional anesthesia, including both spinal and caudal anesthesia, have been proposed as alternatives to general anesthesia to reduce the incidence of postoperative apnea.9
- A multicenter, randomized trial comparing general anesthesia with spinal anesthesia for former preterm and term infants undergoing inguinal herniorrhaphy (GAS trial), showed no difference in the incidence of postoperative apnea (~6% in preterm infants). However, the severity of apnea and the incidence of apnea in the first 30 minutes in the PACU was lower in the spinal anesthesia group.8
- Additionally, the administration of drugs to prolong the duration of a spinal or caudal block such as clonidine or sedative drugs like midazolam or dexmedetomidine are associated with postoperative apnea.
- Therefore, regional anesthesia does not eliminate the need for postoperative monitoring in premature infants.
Postanesthesia Monitoring
- Monitoring vital signs until infants have regained baseline neurologic and cardiorespiratory status is imperative in the PACU.
- Continuous pulse oximetry is necessary because the most common postoperative complication is upper airway obstruction which precipitously leads to hypoxemia.
- Capnography is the quickest way to note if obstruction is occurring; however, most PACUs do not employ this method because monitoring capnography with a natural airway can be challenging in a moving infant.
- The most common dysrhythmia in the PACU is bradycardia due to hypoxemia. Other causes of bradycardia include vagal responses (e.g., nasogastric tube, laryngoscopy), medications (e.g., neostigmine, beta-adrenergic blockade, alpha-2-agonists, opioids such as fentanyl), increased intracranial pressure and high neuraxial anesthetic blockade.
References
- Engle WA; American Academy of Pediatrics Committee on Fetus and Newborn. Age terminology during the perinatal period. Pediatrics. 2004;114(5):1362-4. PubMed
- Eichenwald EC; Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea of prematurity. Pediatrics. 2016;137(1). PubMed
- Erickson G, Dobson NR, Hunt CE. Immature control of breathing and apnea of prematurity: The known and unknown. J Perinatol. 2021;41(9):2111-23. PubMed
- Zhao J, Gonzalez F, Mu D. Apnea of prematurity: From cause to treatment. Eur J Pediatr. 2011;170(9):1097-1105. PubMed
- Cote CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. Anesthesiology.1995;82(4):809-22. PubMed
- Ghazal EA, Vadi MG, Mason LJ, et al. Preoperative evaluation, premedication, and induction of anesthesia. In: Cote and Lerman's a Practice of Anesthesia for Infants and Children. Philadelphia, PA: Elsevier; 2019: 64-66.
- Taenzer A, Havidich J. The postanesthesia care unit and beyond. In: Cote and Lerman's a Practice of Anesthesia for Infants and Children. Philadelphia, PA: Elsevier; 2019:1101-2.
- Davidson AJ, Morton NS, Arnup SJ, et al. Apnea after awake regional and general anesthesia in infants: The general anesthesia compared to spinal anesthesia study- Comparing apnea and neurodevelopmental outcomes, a randomized trial. Anesthesiology. 2015;123(1): 384-54. PubMed
- Nelson O, Muhly WT, Litman RS. The Formerly Premature Infant. In: Litman's Basics of Pediatric Anesthesia. Amsterdam: Elsevier; 2022: 75-76.
- Habib F, Litman RS. The Premature Infant. In: Litman’s Basics of Pediatric Anesthesia. Amsterdam: Elsevier; 2022: 68-69.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.