Copy link
Traumatic Brain Injury
Last updated: 08/17/2023
Key Points
- Traumatic brain injury (TBI) is characterized by an initial primary traumatic insult that results in physical damage to brain parenchyma as well as subsequent secondary injury that can result in cerebral edema, ischemia, and cell death.
- The goal of obtaining an advanced airway in a TBI patient is to pro¬mote airway protection, adequate oxygenation, ventilation, and hemodynamic stability to limit further ischemic injury.
- Efforts to reduce intracranial pressure (ICP) include hyperosmolar therapies, cerebrospinal fluid (CSF) drainage, sedation, therapeutic coma, hyperventilation, therapeutic hypothermia, and/or surgical decompression.
Definition and Classification
- Traumatic brain injury (TBI) is defined as an alteration in brain function or other evidence of brain pathology caused by an external force.1 Common external forces that may result in TBI include:1
- the head being struck by an object;
- the head striking an object;
- acceleration/deceleration of the brain without direct external impact;
- a foreign body penetrating the brain; and
- the force from a blast or explosion.
- TBI comprises an initial traumatic primary insult which results in physical damage to brain parenchyma as well as subsequent secondary injury. Secondary injury can develop over hours to weeks after the initial insult and is a result of multiple ischemic, metabolic, cytotoxic, and inflammatory cascades leading to further neurological injury.2
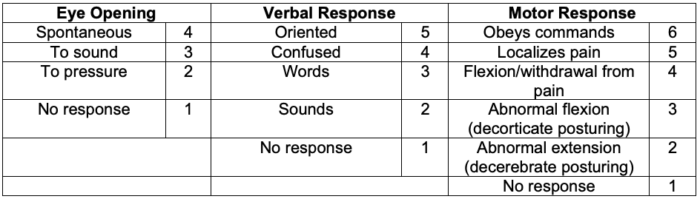
- TBI can be classified using injury severity scores such as the Glasgow Coma Scale (GCS) in addition to neuroimaging. While blood-based biomarkers have recently been approved by the FDA, their clinical utility remains an area of active research.
- Based on the patient’s GCS, TBI can be classified as:
- Mild injury – GCS 13-15
- Moderate injury – GCS 9-12
- Severe injury – GCS less than 8
Anesthetic Concerns
Goals for Anesthetic Management3
- Early cerebral decompression should be facilitated in patients with expanding intracranial hematoma.
- Cerebral perfusion pressure (CPP) should be maintained and increased ICP should be treated.
- Secondary insults such as hypotension, hypoxemia, hyper- and hypocarbia, hypo- and hyperglycemia, seizures, and coagulopathy should be avoided.
- Adequate analgesia and amnesia should be provided.
- Early postoperative neurologic evaluation should be facilitated.
Decision to Obtain an Advanced Airway
- Patients who are comatose or with severe brain injuries (GCS 8 or less) are at an increased risk of respiratory failure due to loss of protective airway reflexes and impaired central regulation of respiratory drive.
- Common criteria for tracheal intubation include:
- hypoxemia not responsive to supplemental oxygen;
- apnea;
- hypercarbia (PaCO2 > 45 mm Hg);
- GCS ≤ 8; and
- a decrease in GCS greater than 3 after initial GCS combined with clinical correlation (anisocoria greater than 1 mm, cervical spine injury compromising ventilation, loss of pharyngeal reflexes, Cushing triad).
- Awake intubation may not be possible due to a lack of patient cooperation.
Monitoring
- Standard American Society of Anesthesiologists monitors should be used and an arterial catheter should be placed. Ideally, the arterial catheter should be placed preinduction to allow for close blood pressure monitoring during induction.
- Central venous access should be considered on a case-by-case basis depending on the patient’s hemodynamic status and medical comorbidities.
Hemodynamic Goals
- To decrease mortality and improve outcomes, the patient’s systolic blood pressure should be maintained ≥ 100 mm Hg for patients 50-69 years of age or at ≥ 110 mmHg for patients 15-49 years or greater than 70 years of age.2
- The recommended target CPP value for survival and favorable outcomes is between 60 and 70 mm Hg.2
Induction of Anesthesia
- All TBI patients should be considered to have a full stomach. Rapid sequence induction (RSI) with manual in-line cervical spine stabilization should be used, as up to 10% of TBI patients also have cervical spine fractures.2,3
- The goal of intubation should be to:
- reduce the risk of pulmonary aspiration while also minimizing further cervical spine trauma;
- maintain adequate cerebral perfusion pressure (CPP), generally greater than 60-70mmg Hg2; and
- avoid extreme shifts in hemodynamics as hypertension may worsen intracranial hemorrhage and elevate ICP, while hypotension can lead to poor cerebral perfusion and ischemia.
Maintenance of Anesthesia
- No anesthetic drug or regimen has been proven to be superior in TBI patients.
- Low-dose inhalational agents and opioids are commonly used for the maintenance of general anesthesia. Total intravenous anesthesia is an alternative for patients at an increased risk for elevated ICPs.
- Benzodiazepines are generally avoided as they may interfere with obtaining frequent neurologic exams.
- Succinylcholine may result in a temporary increase ICP, but can still be used if there are no absolute contraindications.
Fluids and Electrolytes
- Warm, nonglucose-containing isotonic crystalloids should be used to maintain euvolemia. Colloids, such as albumin, are avoided secondary to the concerns of extravasation of albumin from areas of altered blood-brain barrier permeability, leading to cerebral edema.3 Transfusion of blood products should be based on ongoing bleeding, clinical status, and patient comorbidities.3
- The patient’s blood glucose should be closely monitored. While tight glycemic control is controversial, some authors suggest maintaining the patient’s glucose between 80 and 140 mm Hg.3
Seizure Prophylaxis
- Administration of phenytoin is recommended to prevent posttraumatic seizures.2,3 Some clinicians use fospheyntoin or levetiracetam.
- Scheduled anticonvulsants should be continued in the perioperative period.3
Management of Increased ICP
- ICP should be monitored in all severe TBI patients (GCS 8 or less).
- Interventions to lower the ICP are indicated if the ICP is greater than 22 mm Hg in order to maintain a CPP greater than 70 mmHg.2
- Venous drainage should be optimized by elevating the head of the bed to 30°, keeping the neck in a neutral position, and loosening neck braces or airway securing devices around the neck if too tight.
Hyperventilation
- If the blood-brain barrier is intact and cerebral autoregulation is preserved, then hyperventilation can result in cerebral vasoconstriction, which can temporarily decrease ICP but runs the risk of worsening cerebral ischemia as cerebral blood flow (CBF) is reduced.
- For every 1 mmHg decrease in PaCO2, CBF decreases by 3-4%.
- The Brain Trauma Foundation (BTF) guidelines recommend that hyperventilation can be used as a temporizing measure for increased ICP while other more definitive therapies are underway. Prolonged hyperventilation (PaCO2 < 25 mmHg) is not recommended.2
- Continuous hyperventilation may lose its effect as early as 4-6 hours and generally by 20-24 hours as serum and CSF pH will gradually normalize. As PaCO2 levels normalize, arterioles can become dilated and lead to an increase in ICP; therefore, continuous hyperventilation should be discontinued under these circumstances.
Hyperosmolar Therapy
- Both hypertonic saline and mannitol are commonly employed to lower ICP. The BTF does not provide a specific recommendation for hyperosmolar therapy for patients with severe TBI due to insufficient quality of evidence.2
- In severe pediatric TBI, the use of hypertonic saline may be favored over mannitol. ADAPT investigators conducted a comparative effectiveness research study in children with severe TBI and found that a bolus administration of hypertonic saline was associated with lower ICP and higher CPP, whereas mannitol was associated only with higher CPP.4,5 During elevated ICP crises, hypertonic saline was associated with a greater reduction in ICP than mannitol.5
- Serial measurements of electrolytes, serum osmolality and the osmol gap (measured serum osmolality – calculated serum osmolality) should occur every 4-6 hours.
Sedation and Analgesia
- Sedation with propofol, narcotics, and benzodiazepines may be used to decrease CMRO2 and cerebral blood volume via metabolic cerebral blood flow autoregulation.
- Continuous electroencephalography monitoring is often employed to titrate the sedation.
- The clinical utility of barbiturates to lower ICP has been studied in several prospective, randomized controlled trials (RCT) and has failed to demonstrate a clear benefit.2
- While the BTF does not recommend prophylactic barbiturate therapy for intracranial hypertension, if the ICP remains refractory to the maximum medical and surgical therapies, then a barbiturate coma may be indicated with careful hemodynamic monitoring.2
Therapeutic Hypothermia
- Therapeutic hypothermia (32°C to 35°C) has been shown to treat cerebral edema and lower ICP.
- However, RCTs have failed to show a benefit in functional outcomes and caused harm in some studies. Therefore, therapeutic hypothermia is no longer recommended.2,4
CSF Drainage
- An extraventricular drain (EVD) can be used to drain CSF and lower ICP; however, continuous drainage is generally not performed as it can interfere with continuous ICP monitoring.
Surgical Decompressive Craniectomy (DC)
- Either a bifrontal DC or a large frontotemporoparietal DC may be used
- Mortality and favorable outcomes are:6
- improved with DC for LATE refractory ICP elevation
- NOT improved with DC for EARLY refractory ICP elevation
- ICP control and duration of intensive care may be improved by DC for early or late refractory ICP elevation.6
References
- Menon DK, Schwab K, Wright DW, et al. Position statement: definition of traumatic brain injury. Arch Phy Med Rehabil. 2010; 91(11): 1637. PubMed
- Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017; 80(1): 6–15. PubMed
- Sharma D. Anesthesia for patients with acute traumatic brain injury. In: Post T (ed). UpToDate. 2023. Accessed July 20th, 2023. Link
- Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: Update of the Brain Trauma Foundation guidelines. Pediatr Crit Care Med. 2019; 20: S1–S82. PubMed
- Kochanek PM, Adelson PD, Rosario BL, et al. Comparison of intracranial pressure measurements before and after hypertonic saline or mannitol treatment in children with severe traumatic brain injury. JAMA Netw Open. 2022; 5(3): e220891. PubMed
- Hawryluk GWJ, Rubiano AM, Totten AM, et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020; 87: 427–34. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.