Copy link
Hypertrophic Obstructive Cardiomyopathy
Last updated: 08/08/2023
Key Points
- Hypertrophic cardiomyopathy (HCM) is a relatively rare, autosomal dominant myocardial disease with variable genotypic and phenotypic manifestations. It is characterized by left ventricular hypertrophy (LVH) of varying morphologies.
- Asymmetric hypertrophy of the left ventricular (LV) septum can result in obstruction of the LV outflow tract (LVOT), leading to hypertrophic obstructive cardiomyopathy (HOCM).1
- Outpatient management of HOCM is centered around reducing contractility and oxygen demand of the heart with beta-blockers and calcium channel blockers and maintaining normal sinus rhythm with antiarrhythmics such as amiodarone.
- Anesthetic management of HOCM includes maintaining adequate volume status (euvolemia), avoiding increases in contractility and heart rate, and keeping the afterload high (maintaining adequate systemic vascular resistance).
Introduction
- HCM is a relatively rare, autosomal dominant myocardial disease with variable genotypic and phenotypic manifestations. It is characterized by LVH of varying morphologies (Figure 1).
- HCM has several synonyms, including idiopathic hypertrophic subaortic stenosis, muscular subaortic stenosis, and asymmetric septal hypertrophy.1
- Asymmetric hypertrophy of the LV septum can result in obstruction of the LVOT, leading to HOCM.1
- HOCM is often undiagnosed. When patients are symptomatic, they can present with a variety of symptoms, including chest pain, dyspnea on exertion, syncope, or palpitations.
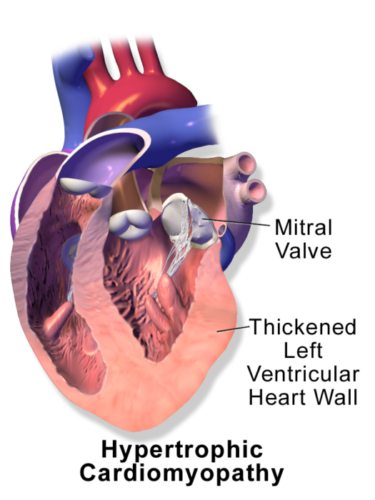
Figure 1. Hypertrophic cardiomyopathy. Source: Wikimedia Commons. Medical gallery of Balusen Medical. 2014. Link.
Epidemiology
- The prevalence of HCM ranges anywhere from 0.2-0.5% in the general population, with symptomatic, obstructive physiology presenting in a small subset of these patients.2
- The multiethnic study of atherosclerosis in 2019 used cardiac magnetic resonance imaging (MRI) to examine 4,972 healthy individuals and found 1.4% (2.6% of men, 0.2% of women) had unexplained LVH, which may suggest previously undiagnosed HCM (given this is a diagnosis of exclusion).3
Presentation
- While the majority of HOCM patients remain asymptomatic, symptomatic patients may present with chest pain, palpitations, presyncope, syncope, and progressive dyspnea on exertion.
- HOCM may also present as acute hemodynamic instability. This may be triggered by physiologic changes that worsen LVOT obstruction (LVOTO), including:
- increases in contractility and/or heart rate (exercise, stress tests),
- decreases in preload (dehydration, hemorrhage), and
- decreases in blood pressure (vasodilating agents, etc.).
Diagnosis
- Physical exam findings of HOCM include a crescendo-decrescendo midsystolic murmur, best heard at the left upper sternal border that is the result of both aortic outflow obstruction as well as mitral regurgitation.
- Mitral regurgitation may be the result of systolic anterior motion (SAM) of the anterior leaflet of the mitral valve, which often accompanies HOCM. SAM is due to the complex interactions of the septal hypertrophy, mitral valve apparatus, and drag forces that lead to obstruction of the LVOT and malcoaptation of the mitral valve leaflets.
- The diagnosis of HOCM has been traditionally confirmed by echocardiography, although cardiac MRI, and to a less effective degree, cardiac computed tomography (CT), is also a useful modality.
- Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) can be used to determine LV morphology, and spectral Doppler (pulsed wave and continuous wave) can be utilized to measure LVOT gradients.
- HCM patients can be classified into 3 categories based on the degree of LOVTO.1

Table 1. Dynamic LVOTO in patients with HCM. Used with permission from Hensley N, et al. Anesth Analg 2015;120(3):554-69.1
Transesophageal Echocardiography
• LV morphology should be assessed, as many different patterns of hypertrophy have been described. Most commonly, a prominent basal septal bulge will be seen in the midesophageal, aortic long-axis view (~120-135°) in the LVOT just below the aortic valve.4
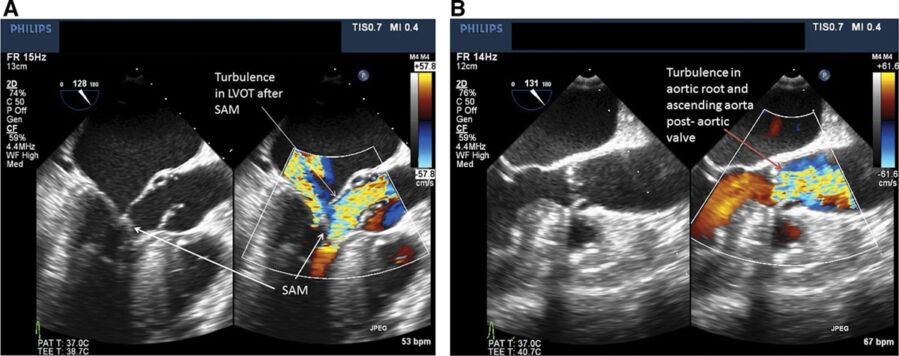
Figure 2. Transesophageal echocardiogram of the mid-esophageal aortic long-axis view between 120-130°. Panel A shows a patient with asymmetric basal septal hypertrophy, turbulence in the LVOT, and systolic anterior motion of the mitral valve, consistent with HOCM. In contrast, panel B shows turbulence in the aortic root and ascending aorta distal to the aortic valve, more consistent with valvular aortic stenosis. Used with permission from Hensley N, et al. Anesth Analg 2015;120(3):554-69.1
-
- LVOT gradients should be assessed at rest (or under anesthesia) as well as with provocation. HOCM is a dynamic process that may be elucidated with interventions such as a Valsalva maneuver, administration of inotrope, or administration of a vasodilating agent.
- The septal hypertrophy results in narrowing of the LVOT and leads to turbulent flow and a late-peaking “dagger” shape on spectral Doppler.
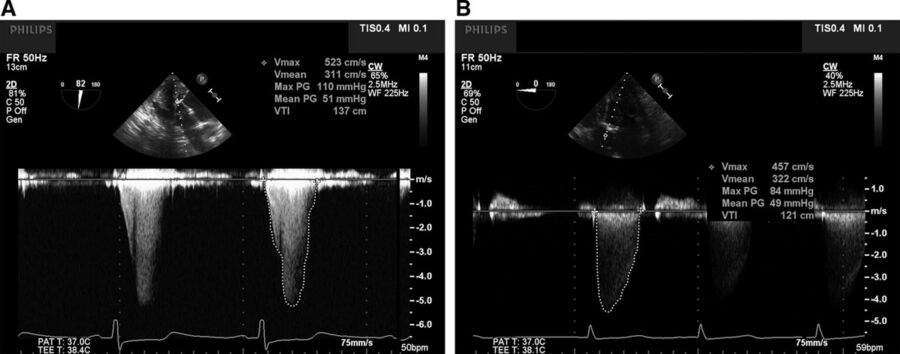
Figure 3. Panel A shows the characteristic mid- to late-systolic peaking of the continuous wave Doppler spectral profile across the LVOT (“dagger-shaped”) in the deep transgastric transesophageal echocardiogram view, consistent with subvalvular, dynamic LVOTO. Panel B, in contrast, shows the continuous wave Doppler spectral profile of a patient with severe aortic stenosis with fixed obstruction. Note the early systolic peaking parabolic shape. Used with permission from Hensley N, et al. Anesth Analg 2015;120(3):554-69.1
- Premyectomy TEE should focus on:
- Septal thickness measurements:
- Maximal septal thickness
- Distance from the right coronary cusp (RCC) to maximal thickness
- Distance from RCC to distal narrowing
- Mitral valve morphology
- Presence (or risk factors) of SAM
- Small LV cavity less than 4.5 cm
- C-Sept distance less than 2.5 cm
- Anterior: posterior mitral leaflet height ratio less than 1.3
- Aorto-mitral angle less than 120°
- LVOT peak gradient
- LV function
- Septal thickness measurements:
- Indications for surgical intervention include SAM physiology or an LVOT peak gradient greater than 50 mmHg (at rest or provoked).
- The postmyectomy TEE exam should again include the septal thickness measurements, LVOT diameter, evaluation for residual SAM, peak LVOT gradient, and evaluation of a possible ventricular septal defect (VSD).5
Treatment Options for Patients with HCM
- Given the risk of sudden cardiac death, patients with HCM should be managed either medically or procedurally. Pharmacologic treatment options for HCM include beta-blockers, calcium channel blockers, and disopyramides, all of which decrease gradients across the LVOT.1
- Alcohol septal ablation uses ethanol injection into the septal perforator arteries of the left anterior descending (LAD) coronary artery to induce septal infarction and basal thinning and to reduce the LVOT gradient.
- Good candidates for alcohol septal ablation include those who have continued symptoms despite pharmacological treatment, septal thickening ≥15-16 mm, LVOT gradient of ≥ 30 mmHg at rest or ≥ 50 mmHg on provocation, favorable septal artery anatomy, and absence of mitral valve abnormalities or coronary artery disease.
- Surgical myectomy involves resection of the septal muscle.
- Good candidates for surgical myectomy include the same criteria as alcohol septal ablation as well as those who have concomitant mitral valve disorders, coronary artery disease, or other intracardiac lesions.
- Surgical myectomy can be complicated by ventricular septal defects as well as conduction defects.
Anesthetic Management of HCM Patients Undergoing Noncardiac Surgery
Preoperative Evaluation
- The preoperative evaluation should focus on assessing the severity of disease, functional status, cardiac and respiratory symptomatology, personal and family cardiac history, current medications, history of rhythm disturbances, previous strokes, or congestive heart failure.1
- During the physical examination, murmurs should be evaluated for dynamic changes. Valsalva maneuver, standing, and exercise increase the intensity of the murmur, and conversely, squatting and beta-blockade decrease the intensity of the murmur.
- A baseline ECG should be performed to monitor for intraoperative changes.
- Preoperative echocardiographic assessment should include the determination of abnormalities of the mitral valve and subvalvular apparatus, LVOT gradients, LV systolic function, degree of diastolic dysfunction, etc.
- Echocardiographic red flags for HCM patients include:1
- significant LVOT pressure gradients (greater than 30 mm Hg at rest or greater than 50 mm Hg during provocation);
- moderate to severe mitral regurgitation due to SAM of the mitral valve;
- depressed LV systolic function with ejection fraction less than 45-50%; and
- restrictive diastolic dysfunction by transmitral Doppler flow (TMDF) and pulmonary venous Doppler flow velocity (PVDF) profile.
- Early-to-late TMDF velocities (E/A greater than 1.7)
- Isovolumic relaxation time less than 90 milliseconds
- Deceleration time less than 140 milliseconds
- PVDF systolic velocity to diastolic velocity less than 0.8.
- Patients should be instructed to continue beta-blockers, calcium channel blockers, and disopyramide. They should also be encouraged to remain hydrated perioperatively.
Intraoperative Management
- The hemodynamic goals for HCM are similar to aortic stenosis (Table 2). The significant difference is the dynamic nature of the LVOTO. Increases in heart rate, rhythm disturbances, and decreases in afterload will exacerbate LVOTO. Additionally, increases in contractility and decreases in preload will accentuate LVOTO.1
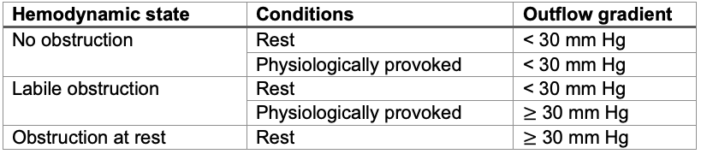
Table 2. Hemodynamic goals in patients with HCM
- In addition to standard American Society of Anesthesiologists monitors, an intraarterial catheter and/or noninvasive pulse plethysmographic variability index monitoring and central venous pressure monitoring should be considered. For high-risk patients undergoing major surgery, a TEE should be considered.1
- If a neuraxial anesthetic is performed, slow controlled titration of medication via an epidural is preferred over a spinal to maintain preload and afterload and avoid sympathetic stimulation.1
- Great care must be taken during induction of general anesthesia to minimize exacerbation of LVOTO. Fluid loading should be considered to ensure adequate preload and avoid hypovolemia. Diuretics should be used cautiously for this reason.
- If possible, inotropes such as epinephrine or calcium should be avoided, and normotension should be maintained.
- Phenylephrine should be considered over epinephrine, norepinephrine, or ephedrine as it results in a more favorable hemodynamic profile (increased afterload, decreased heart rate, and lack of inotropy).
- Sympathetic stimulation from intubation, surgical stimulus, blood loss, pain, light anesthesia, etc. can precipitate hemodynamic collapse.1
- Normal sinus rhythm should be maintained perioperatively because LVH and decreased LV compliance necessitate the increased dependence on atrial kick for maintaining cardiac output. Arrhythmias that decrease ventricular filling, such as atrial fibrillation, should be aggressively treated. Cardioversion may be necessary for hemodynamically unstable atrial fibrillation.
References
- Hensley N, Dietrich J, Nyhan D, et al. Hypertrophic cardiomyopathy: A review. Anesth Analg. 2015;120(3):554-69. PubMed
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation.1995; 92(4):785-9. PubMed
- Massera D, McClelland RL, Ambale-Venkatesh B, et al. Prevalence of unexplained left ventricular hypertrophy by cardiac magnetic resonance imaging in MESA. J Am Heart Assoc 2019; 8: e012250. PubMed
- Nagueh SF, Phelan D, Abraham T, et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2022; 35(6):533-569. PubMed
- Vegas A. Perioperative two-dimensional transesophageal echocardiography: A practical handbook: Second edition. Springer International Publishing. 2018.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.