Copy link
Aneurysmal Subarachnoid Hemorrhage Part 2: Management and Anesthetic Considerations
Last updated: 07/18/2023
Key Points
- Aneurysmal subarachnoid hemorrhage (aSAH) is a neurologic emergency.
- Elevated blood pressure on presentation is associated with greater hematoma expansion, neurologic deterioration, death, and dependency.
- Surgical clipping of the aneurysm or endovascular coiling are the main treatment options.
- Key aspects of anesthetic management include smooth induction and emergence with strict blood pressure control, control of intracranial pressure (ICP) and brain swelling, maintenance of adequate cerebral perfusion pressure (CPP).
- High blood loss can occur especially during uncontrolled intraoperative aneurysm rupture.
- The mainstay of critical care management includes maintenance of adequate CPP, prevention of brain herniation and monitoring and treatment of cerebral vasospasm to prevent delayed cerebral ischemia.
Early Management
- The initial management of patients with aSAH should focus on stabilizing life-threatening conditions, optimizing cerebral perfusion, and minimizing further neurological injury.1,2
Maintenance of Oxygenation and Ventilation
- Adequate oxygenation, ventilation, and a patent airway should be ensured.
- Hypoxia worsens brain injury and should be avoided. Similarly, both hypo- and hypercarbia result in poor neurological outcomes. Hypoxia induces cerebral vasoconstriction and ischemia, and hypercarbia causes cerebral vasodilation, resulting in intracranial hypertension and decreased cerebral perfusion.1
- Indications for tracheal intubation include1,2
- Comatose patient with Glasgow coma scale (GCS) ≤ 8
- Hypoxia or hypoventilation
- Hemodynamic instability
- Deep sedation or general anesthesia needed for imaging.
Restoration of Cerebral Perfusion
- Early placement of an external ventricular drain (EVD) allows lowering the raised intracranial pressure (ICP) and restoring cerebral perfusion. It also facilitates surgical exposure during aneurysm clipping.1
- Common thresholds for EVD placement include1
- Ventriculomegaly in patients with GCS ≤ 12
- Hunt and Hess grade ≥ 2
Blood Pressure Control
- Strict blood pressure control is critical. Systolic blood pressure less than 160 mm Hg or mean arterial pressure less than 110 mm Hg should be maintained. Common pharmacological choices include intravenous nicardipine, esmolol, labetalol, and celvidipine.1,2 Nitroprusside should be avoided secondary to the risk of increased cerebral blood volume and ICP.
- Hypotension should be avoided as well.
Nimodipine
- Nimodipine 60 mg orally or via a nasogastric tube every 4 hours has been demonstrated to improve outcomes in aSAH and is considered a standard of care.1,2
- Nimodipine should be started within 48 hours of aSAH and continued for 21 days.
- Possible mechanisms for its neuroprotective benefits include1,2
- dilation of small arteries not visible on angiograms;
- reduction of calcium-dependent excitotoxicity; and
- decreased platelet aggregation.
Reversal of Anticoagulation
- All antithrombotic agents should be discontinued and anticoagulation reversed as needed.
- See acute subdural hematoma summary for details.
Seizure Prophylaxis
- The prophylactic use of antiseizure medications after aSAH is controversial as it has been associated with worse neurologic and cognitive outcomes.1,2
General Supportive Measures
- Adequate analgesia for headache and neck pain
- Maintenance of euvolemia with crystalloids and monitoring urine output
- Deep vein thrombosis prophylaxis with intermittent pneumatic compression devices
Treatment Options
- The main treatment options for aSAH are surgical clipping and endovascular coiling.
- Surgical clipping via a craniotomy involves placing a clip across the neck of the aneurysm (Figure 1). Surgical clipping is preferred in patients with large intraparenchymal hematomas, aneurysm of the middle cerebral artery, and patients who are not likely to be compliant for long-term follow-up.1
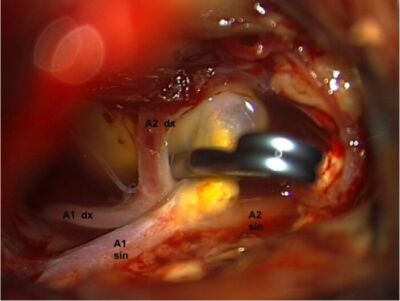
Figure 1. Surgeon's view of craniotomy and clipping of anterior cerebral artery aneurysm. A titanium clip was placed across the neck of the aneurysm after isolating parts of the left (A1 sin & A2 sin) and right (A1dx & A2dx) anterior cerebral arteries. Source: Wikimedia Commons. Roberto Stefini. CC BY-SA 4.0 Link.
- Endovascular techniques involves placing coils into the lumen of the aneurysm, which promotes thrombus formation and obliteration of the aneurysm sac (Figure 2). Other options include stent-assisted coiling, balloon-assisted coiling, etc.
- Endovascular treatment is usually preferred in geriatric patients, especially those presenting with high-grade aSAH from the rupture of basilar apex aneurysms.1
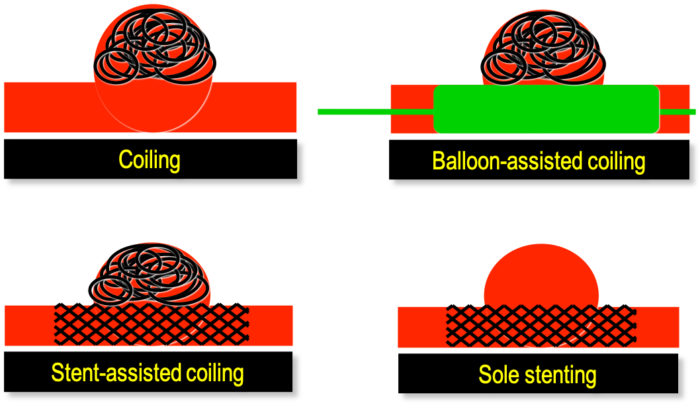
Figure 2. Different endovascular techniques to treat an intracranial aneurysm. Depending on the size, anatomy, and location on the parent vessel of the aneurysm various coiling and stenting strategies can be utilized. Source: Wikimedia Commons. DocJBrigham, CC BY-SA 4.0 Link.
Anesthetic Considerations for Surgical Clipping
- The main anesthetic goals during surgical aneurysm clipping are the following.1
- Preventing rebleeding
- Maintaining cerebral perfusion
- Facilitating surgical exposure by preventing/managing brain swelling
- Facilitating intraoperative neurophysiologic monitoring (IONM)
- Facilitating temporal aneurysm clipping
- Anticipating and managing aneurysm rupture
- Ensuring smooth emergence and neurologic assessment
Induction of Anesthesia
- The primary goal is avoiding hypertension in response to laryngoscopy and intubation, as it increases the risk of rebleeding. Strategies include ensuring adequate anesthetic depth prior to laryngoscopy, using short-acting opioids or antihypertensive agents along with induction drugs.1
- Hypotension following induction increases the risk of cerebral ischemia and should be aggressively treated with vasopressors.
- A preinduction arterial line allows continuous blood pressure monitoring and should be considered in most patients, especially in patients with cardiac dysfunction, elevated troponin levels, and hemodynamic instability.
- Hypo- and hypercarbia should be avoided during bag-mask ventilation. Succinylcholine may be used for rapid sequence induction, if needed.1
Intraoperative Monitoring
- In addition to standard American Society of Anesthesiologists monitors, an arterial line allows continuous blood pressure monitoring and frequent measurement of arterial blood gases, glucose, and electrolytes.
- Ideally, the arterial line should be inserted prior to induction. It may be deferred until postinduction for patient comfort or team preference in patients who are at low risk for aneurysm rupture.
- Placement of a central venous catheter is usually not needed unless the postoperative infusion of vasopressors and inotropes is anticipated. Large bore peripheral vascular access should be secured in preparation for high blood loss.
- ICP and cerebral perfusion pressures (CPP) should be monitored if an external ventricular drain is present.
- IONM including somatosensory-evoked potentials and motor-evoked potentials may be used for the early detection of cerebral ischemia and dictates the choice of anesthetic agents.
- Electroencephalogram (EEG) monitoring may be needed if burst suppression is needed during temporary clipping.
Anesthesia Maintenance
- The choice of anesthetic agents depends on the patient’s neurological status, comorbidities, treatment approach (surgical clipping vs. endovascular coiling), and the need for IONM.
- A balanced anesthetic technique with both inhaled and intravenous agents is commonly used.
- Propofol maintains the coupling between cerebral metabolic oxygen rate of oxygen (CMRO2) and cerebral blood flow (CBF) while inhaled anesthetics have a dose-dependent effect on CBF. At less than 1 MAC, inhaled anesthetics decrease CBF, while they cause cerebral vasodilation at higher doses, resulting in uncoupling of CMRO2 and CBF. The cerebral vasodilatory effects of inhaled anesthetics can be minimized by hyperventilation.
Hemodynamic Goals
- As mentioned previously, hypertension should be avoided before the aneurysm is secured. Critical periods include placement of skull pins, patient positioning, and surgical stimulation.
- Hemodynamic goals include maintaining SBP less than 160 mm Hg, MAP less than 110 mm Hg, and CPP greater than 60-70 mm Hg.1,2
- During temporary clip placement, the patient’s blood pressure should be raised by 10-20% to ensure perfusion through collateral vessels.1 Blood pressures can be normalized after the aneurysm is secured.
ICP Management and Brain Relaxation
- To facilitate surgical exposure, strategies for intraoperative brain relaxation include:
- Maintaining an adequate depth of anesthesia and analgesia
- Optimizing hemodynamic parameters
- Optimizing patient positioning to facilitate cerebral venous drainage
- Maintaining normocarbia to moderate hypocarbia (PaCO2 30-35 mm Hg). Brief hyperventilation to PaCO2 of less than 30 mm Hg may be used if other ICP reduction maneuvers fail.
- Hyperosmolar therapy such as intravenous mannitol, furosemide, or hypertonic saline
- Cerebrospinal fluid (CSF) drainage via an external ventricular drain
Temporary Clipping
- A temporary clip may be placed on the feeding vessel to facilitate dissection and accurate placement of a permanent clip around the neck of the aneurysm. Clip application for a duration of up to 10 minutes is usually well tolerated without ischemia of the middle cerebral artery territory.1
- Strategies to prevent ischemic damage during temporary clipping include:
- Inducing temporary hypertension to promote collateral flow (10-20% increase in SBP)
- Avoiding prolonged temporary clipping (usually greater than 10 minutes)
- Reducing CMRO2 using burst suppression
- Using IONM to detect ischemia and alert a signal change
- Intraoperative hypothermia for neuroprotection is not recommended.3 Similarly, hyperthermia should be avoided as well.
- Intraoperative hyperglycemia worsens neurological outcomes and should be avoided. While no specific threshold glucose values are advised, periodic glucose monitoring and maintaining blood glucose between 80 and 180 mg/dL is recommended.1
Adenosine-Induced Temporary Flow Arrest
- Adenosine is useful in controlling bleeding if the aneurysm ruptures intraoperatively before clipping. The induced flow arrest provides a clear surgical field and allows the surgeon to control the source of bleeding.
- Adenosine is a negative dromotropic and chronotropic agent with rapid onset and short duration of action that causes hypotension and can cause a brief asystole of variable duration. A dose of 0.3–0.5 mg/kg leads to approximately 1 minute of moderate hypotension.1
- Recovery of post arrest may be preceded by transient cardiac arrhythmias including atrial fibrillation, ventricular tachycardia, or atrial flutter, and is best avoided in patients with coronary artery disease or abnormalities of the cardiac conduction system.
- Adenosine may also cause bronchospasm and in patients with reactive airways disease.
- Intraoperative ICG video or conventional angiography are useful modalities to check the status of the aneurysm and the patency of nearby arteries after clipping.
Emergence
- A smooth emergence from general anesthesia without coughing, straining, and hypertension should be ensured.
- Coughing and straining increases intrathoracic pressure and transient increases in both cerebral arterial and venous pressure which can cause edema, bleeding, and increase in ICP.
- Short-acting opioids can be titrated to spontaneous ventilation towards the end of the procedure. Remifentanil or dexmedetomidine infusions may be continued throughout emergence to reduce airway reactivity.
- The use of nitrous oxide to facilitate emergence is not recommended given the increased risks of pneumocephalus and postoperative nausea and vomiting.
- Lidocaine administration (1-1.5 mg/kg IV) is effective for reducing airway reflexes.
- Hypertension increases the risk of intracerebral hemorrhage after craniotomy and should be aggressively treated.
- Betablockers: labetalol and esmolol (Caution is advised in stress-induced cardiomyopathy.)
- Calcium channel blockers and vasodilators: diltiazem, nicardipine, clevidipine, and hydralazine
- Dexmedetomidine used during the procedure or prior to closing can reduce the response to emergence and hypertension in the PACU.
- Deferring extubation should be discussed with neurosurgical team.
- Indications for postoperative ventilation include but are not limited to preoperative intubation, low preoperative GCS, extremely long surgery (greater than 8-10h), posterior circulation and posterior cranial fossa or close to the brain stem, or a high risk for the development of cerebral edema.
- The anesthetic is still weaned off to allow a clinical neurologic evaluation.
- In patients where a planned extubation cannot be performed within 1-hour postsurgery, an immediate head computed tomography should be performed.
Anesthetic Considerations for Endovascular Coiling
- The anesthetic goals for endovascular techniques (e.g., coiling or flow diversion stent) are similar to surgical clipping, with the following exceptions.1
- IONM is not commonly used.
- Interventions to reduce brain swelling are not needed.
- Patient immobility, especially during coil deployment is critical.
- Heparin is commonly administered for anticoagulation and may need to be reversed.
- Although monitored anesthesia care may be used for diagnostic angiography, general anesthesia (GA) is usually preferred for endovascular coiling.
- Advantages of using GA include:4
- Immobility during the procedure
- Patient comfort during stimulating procedure
- Easier to control comorbidities or neurologic condition of the patient
- Airway protection and control of ventilation (PaCO2 manipulation, apnea)
- Disadvantages of GA include:4
- Hemodynamic instability during intubation, extubation, or both
- Anesthetic-induced hypotension causing cerebral hypoperfusion
- Inability to monitor the patient’s neurologic status
- Complications from coughing and straining during extubation
- Delayed emergence or impaired neuro exam due to residual anesthetic drugs
- With GA, the same requirements for induction/intubation and emergence/extubation apply as during open craniotomy for aneurysmal clipping.
Early Complications
Rebleeding
- Patients with aSAH are at an increased risk for early rebleeding (4-14% in the first 24 hours).2
- Rebleeding is characterized by an acute deterioration of neurological status accompanied by the appearance of new hemorrhage on the head CT scan.
- To reduce the risk of rebleeding, the ruptured aneurysm should be treated via surgical clipping or endovascular coiling as soon as possible and ideally, within the first 24 hours after symptom onset.
Vasospasm and Delayed Cerebral Ischemia
- Cerebral vasospasm is a devastating complication of aSAH that typically occurs between 3 and 14 days posthemorrhage and result in delayed cerebral ischemia.1,2
- Please see the summary on cerebral vasospasm.
Hydrocephalus and Elevated ICP
- Hydrocephalus affects 20-30% of patients with aSAH from obstruction of CSF flow and decreased CSF absorption.2,4
- CSF diversion via an external ventricular drain is often needed.
- About 20% of patients need long term CSF diversion (ventriculoperitoneal shunt) for chronic hydrocephalus
Hyponatremia
- Hyponatremia occurs in up to 30% of patients with aSAH.2-4
- Hyponatremia after SAH is more likely due to cerebral salt-wasting syndrome (CSW) from release of natriuretic peptide than syndrome of inappropriate secretion of antidiuretic hormone (SIADH).3,4
- CSW ➞ triad of hyponatremia, volume contraction, and high urine Na+ concentrations (>50 mmol/L); correlated with symptomatic vasospasms.
- SIADH ➞ normovolemia or mild hypervolemia; treated by volume restriction.
- Management ➞ Administration of isotonic and/or hypertonic fluids using intravascular normovolemia and normonatremia as the end point.
Hypernatremia
- Cause is often iatrogenic related to hyperosmolar therapy (hypertonic saline or mannitol).
- Central diabetes insipidus can be caused by hypothalamic/pituitary ischemia from delayed cerebral ischemia related to vasospasm or reduced cerebral perfusion in the setting of intracranial hypertension. Hypernatremia in the subacute phase of SAH is associated with poor outcomes.5
- Antidiuretic hormone is synthesized in the supraoptic nuclei of the hypothalamus and transported to the supraoptic-hypophyseal tract in the posterior lobe of the pituitary gland.
- Diabetes insipidus rarely arises intraoperatively and usually occurs 12 – 48 hours postoperatively.
- Clinical picture includes polyuria with a rising serum sodium and serum osmolality.
- Diagnosis is made when urine output is greater than 250 mL for 2 hours, Na is greater than 140, and urine osmolarity is low or urine specific gravity is < 1.005.
- Treatment includes intravenous DDAVP (deamino D-arginine vasopressin) (1-2 mcg) and volume repletion.
Cardiac Complications
- Serious cardiac complications associated with SAH include stress-induced cardiomyopathy and associated arrhythmias. Please see summary on aneurysmal subarachnoid hemorrhage part 1.
- Supportive measures include enhanced invasive cardiovascular monitoring (echocardiography, pulmonary artery catheter, etc), cardiovascular medications such as inotropes, vasopressors, and antiarrhythmogenic medications.
References
- Sharma D. Perioperative management of aneurysmal subarachnoid hemorrhage: A narrative review. Anesthesiology. 2020; 133:1283–1305. PubMed
- Singer RJ, Ogilvy CS, Rordorf G. Aneurysmal subarachnoid hemorrhage: Treatment and prognosis. In: Post T (ed). UpToDate. 2023. Link
- Todd MM, Hindman BJ, Clarke WR, et al. Intraoperative hypothermia for aneurysm surgery trial (IHAST) investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352(2):135-45. PubMed
- Lemkull BP, Drummond JC, Patel PM, et al. Anesthesia for neurologic surgery and neurointerventions. In: Gropper MA, Miller RD (eds). Miller's Anesthesia. 9th Edition. Elsevier, Philadelphia, PA, Elsevier. 2020. 1868–1910.
- D'Souza S. Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2015;27(3):222-40. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.