Copy link
Local Anesthetic Systemic Toxicity
Last updated: 03/22/2023
Key Points
- While local anesthetic systemic toxicity (LAST) typically presents with prodromal symptoms followed by possible progression to more severe neurologic and then cardiovascular complications, there is an increasing trend towards atypical presentations.
- Simple preventative steps, the use of ultrasound guidance for peripheral nerve blocks, and vigilant monitoring are important for reducing the likelihood of LAST.
- The treatment of LAST prioritizes early airway management, rapid administration of intralipid therapy, seizure control, and effective cardiopulmonary resuscitation.
- LAST occurs from the combination of inhibition of Na, K, Ca, and N-methyl-D-aspartate (NMDA) channels, interference of metabolic signaling, and inhibition of intracellular energy production.
- Risk factors for LAST include patient factors, pharmacologic factors, and procedure factors.
Introduction
- LAST is a potentially life-threatening adverse reaction resulting from circulating levels of local anesthetic reaching toxic levels after administration.
- There is wide variation in published incidences of LAST, but two recent studies of different administrative databases have estimated the overall rate to be approximately 1-2 events per 1000 peripheral nerve blocks in total joint arthroplasty patients who received a peripheral nerve block.1,5
- Other studies based on registries, national surveys, and single-institution data have shown a lower incidence of LAST (<1/1000). This may reflect different definitions of LAST, as well as a lower incidence in highly specialized academic centers, where high case volumes and the use of ultrasound are more prevalent.
- LAST occurs less frequently in the pediatric population, perhaps due to most regional anesthesia being performed under general anesthesia (which raises the seizure threshold), smaller local anesthetic volumes, and children having fewer comorbidities.
- While the incidence of LAST appears to be decreasing in recent studies, there is a high likelihood of under-reporting and misdiagnosis. Due to the potential morbidity and mortality associated with LAST, more awareness is important in reducing the likelihood of LAST and ensuring optimal management.
Presentation
- Central nervous system (CNS) toxicity is the most common feature of LAST, primarily in the form of seizures.2
- The classic presentation consists of symptoms occurring soon after the injection of the local anesthetic, with initial CNS excitation (perioral numbness, metallic taste, tinnitus, blurry vision, dizziness, confusion, agitation, tremors), followed by seizures, and possible progression to CNS depression (lethargy, coma, respiratory arrest).1,3
- Further progression can occur to cardiovascular (CV) toxicity. CV symptoms can include (in decreasing order of frequency): dysrhythmias, conduction delay, cardiac arrest (most commonly asystole), and bradycardia/hypotension.1,2
- An increasing number of patients (40%) present atypically with LAST.2 Recent data has shown that patients often have CV signs alone (about 24% of the time, there is no evidence of CNS toxicity), present with both CNS and cardiac toxicity simultaneously, and/or have a delayed presentation (more than 5 minutes after injection of the local anesthetic).1
- The increasing frequency of delayed onset of LAST could reflect fewer intravascular injections due to ultrasound guidance, increasing use of local tissue infiltration techniques, and increasing use of continuous local anesthetic infusions. LAST has been described to have occurred 30 minutes or even longer after the injection of the local anesthetic.
- There is a trend towards delayed-onset LAST outside of the operating room (20%), and this may present with more subtle manifestations (i.e., instead of CV collapse, refractory relative hypotension).3 There is also an increasing trend towards events involving nonanesthesia trained practitioners (up to 50% of cases), often after simple tissue infiltration.
Pathophysiology
- LAST occurs when an excess of local anesthetic unbound in the plasma and tissues binds and inhibits Na channels in neurons in the brain and cardiac myocytes causing dysfunction of each.2
- Additional abnormalities may ensue with excess local anesthetic at increasing serum concentrations through blockade of K, Ca, and NMDA channels.4
- At very high serum levels of local anesthetics, many metabolic derangements occur at the cellular level, including interference of mitochondrial oxidative phosphorylation, free fatty acid utilization, and cyclic AMP production.1
- Oxidative phosphorylation can be extensively inhibited by local anesthetics.
- It is the total combination of derangements of channel blockade, metabolic signaling, and intracellular energy production that leads to the negative effects upon the organs least able to cope with such insults, the brain, and the heart.
Risk Factors
Risk factors for LAST include patient factors, pharmacologic factors, and procedural factors.1,5
Patient Factors
- Extremes of age—younger than 16 or more than 60 years
- Low muscle mass—neonates, infants, frail elderly patients
- Females more than males
- Pregnancy (increased sensitivity to local anesthetics, increased cardiac output, epidural venous engorgement, decreased protein binding)
- Cardiac disease—conduction abnormalities, ischemic heart disease, and congestive heart failure
- Liver disease
- Metabolic disease—diabetes, isovaleric acidemia, mitochondrial disease, and carnitine deficiency
- Central nervous system (CNS) disease
- Low plasma protein binding states—liver disease, malnourishment, infants, pregnancy
Pharmacologic Factors
- Certain local anesthetics have a narrower CNS-to-cardiac ratio of toxicity, leading to earlier onset of severe cardiac toxicity relative to the onset of CNS toxicity, owing to differences in affinity for myocardial sodium, potassium, and calcium channels (e.g., bupivacaine). However, LAST can occur with any local anesthetic.6
- Total local anesthetic dose and total dose per kilogram of patient body weight are important pharmacologic factors leading to toxic levels of local anesthetic in plasma and tissues.
- Block site, total local anesthetic dose, and patient comorbidities are more predictive of high plasma levels of local anesthetics than body weight or body mass index.1
- Continuous local anesthetic infusions are at higher risk after 1-4 days of an infusion, as plasma levels may continue to rise.
Procedural Factors
- Ultrasound guidance reduces the risk of LAST relative to other localization methods.7
- Peripheral nerve blocks have an increased risk of LAST relative to neuraxial blockade.
- Among peripheral block locations, risk is thought to relate most to the vascularity of surrounding tissues and absorption characteristics such that lower limb blocks have less risk than upper limb blocks, which have less risk than paravertebral blockade.
Practice Setting
- Up to 20% of LAST cases occur outside the hospital setting.1
- Nonanesthesiologists are involved in up to 50% of LAST cases.
Prevention
While no single measure can eliminate the risk of LAST, several preventive steps have been proposed.
- The lowest effective dose of local anesthetics (individualized based on body mass, site of injection, and patient’s age and medical history) should be used.1 In obese patients, dosing the local anesthetic based on lean body weight should be considered.
- Incremental injections of local anesthetics should be used by administering 3-5 mL aliquots and pausing 15-30 seconds between injections. Ideally, the time between injections should include one circulation time (~30-45 seconds), which may be increased with lower extremity blocks and inpatients with decreased cardiac output.
- Before each injection, consider aspirating even though this intervention has ~ 2% false negative rate.
- Using an intravascular marker such as epinephrine should be considered, recognizing that epinephrine is an imperfect marker.
- In adults, intravascular injection of epinephrine 10-15 mcg/mL increases heart rate by at least 10 bpm and systolic blood pressure by at least 15 mm Hg. These hemodynamic responses are attenuated in the presence of beta-blockade, elderly patients, and patients who are sedated or under general anesthesia. Its use in labor patients is also controversial secondary to the false-positive response from uterine contractions.1,6
- In children, intravascular injection of epinephrine 0.5 mcg/kg increases systolic blood pressure by at least 15 mmHg.
- Ultrasound guidance significantly reduces the risk of LAST by up to 65% during peripheral nerve blocks.
- Clinicians should be aware of the additive nature of local anesthetic toxicity, especially when combining local anesthetics, redosing local anesthetics, or when administered by different perioperative providers.
- During truncal blocks, a lower concentration of local anesthetics should be used, dosing on lean body weight, and epinephrine should be used as a marker. Clinicians should observe for at least 30-45 minutes after the block.1
- The increased risk of LAST should be recognized in certain patients (young age, patients, patients with renal, hepatic, or cardiac disease, malnourished patients, etc.).
- Including the maximum local anesthetic dosing in the surgical time-out should be considered.
- Vigilant monitoring remains critical, and patients should be monitored at least 30 minutes after the injection of potentially toxic doses of local anesthetic.2
Treatment
- The treatment of LAST is different from conventional resuscitation because local anesthetic cardiotoxicity differs pathophysiologically from other causes of CV collapse.1
- Instead of prioritizing rapid cardiac support, successful LAST treatment depends on early airway management to prevent hypoxia, hypercapnia, and acidosis. These conditions are known to potentiate LAST and prevent resuscitation, perhaps due to increased free fraction of local anesthetic and/or worsening of cardiac function.
- As soon as possible after airway management, early infusion of lipid emulsion is recommended as evidence exists that lipid shuttling is most effective if given early in the LAST event and adverse effects of administration are minor.2
- Immediate management begins with stopping further local anesthetic injection and calling for assistance. Effective airway management, ventilation, and circulation should be addressed, and lipid emulsion administered.
- The goal should be normocapnia, as hypercarbia increases cerebral blood flow (increasing local anesthetic delivery to the brain), and hyperventilation reduces cardiac output.8
- Infusing lipid emulsion reverses LAST by accelerating the redistribution of the local anesthetic.
- This results from partitioning and a direct inotropic effect exerted by lipid emulsion, which combine to “shuttle” the local anesthetic away from sensitive organs (brain, heart) to reservoir organs (skeletal muscle, liver).9
- It is important to infuse a relatively large quantity of lipid quickly (e.g., 1.5 mL/kg bolus) to establish a lipid “bulk phase” in the plasma. The bolus may be repeated (may be superior) or followed by a slower infusion (0.25 mL/kg/min), to sustain a bulk phase. The dose should be limited to about 12 mL/kg ideal body weight to avoid dangerous lipid overload.1
- Seizures should preferably be controlled with benzodiazepines. Small doses of propofol can be used with caution due to its cardiodepressant effects. For refractory tonic-clonic muscle contractions, neuromuscular blockers can be considered as ongoing muscle contraction further worsens hypoxia and acidosis.8
- Conventional cardiopulmonary resuscitation (CPR) can be employed, but smaller than usual doses of epinephrine should be given (≤1 mcg/kg) because full 1mg doses can worsen arrhythmias, impair pulmonary gas exchange, increase afterload and plasma lactate, and interfere with lipid resuscitation.
- Vasopressin increases afterload to the potentially weakened heart and may worsen outcomes in animal studies of LAST.
- If ventricular arrhythmias develop, amiodarone is preferred (do not give local anesthetics such as lidocaine or procainamide).
- Effective CPR to reduce myocardial local anesthetic concentrations is very important, as evidence suggests that this is critical for the ionotropic benefit of intralipid to occur.1
- CPR should be continued for a prolonged period, if necessary (at least 60 minutes), as good neurological recovery can occur even after long cardiac arrests secondary to LAST.
- Consider the need for extracorporeal methods of circulatory support early during severe cases.9
- It is advisable to monitor LAST patients who have experienced a significant cardiovascular event for at least 4-6h after treatment. If the event is limited to CNS symptoms, it may be prudent to monitor for at least 2h.1
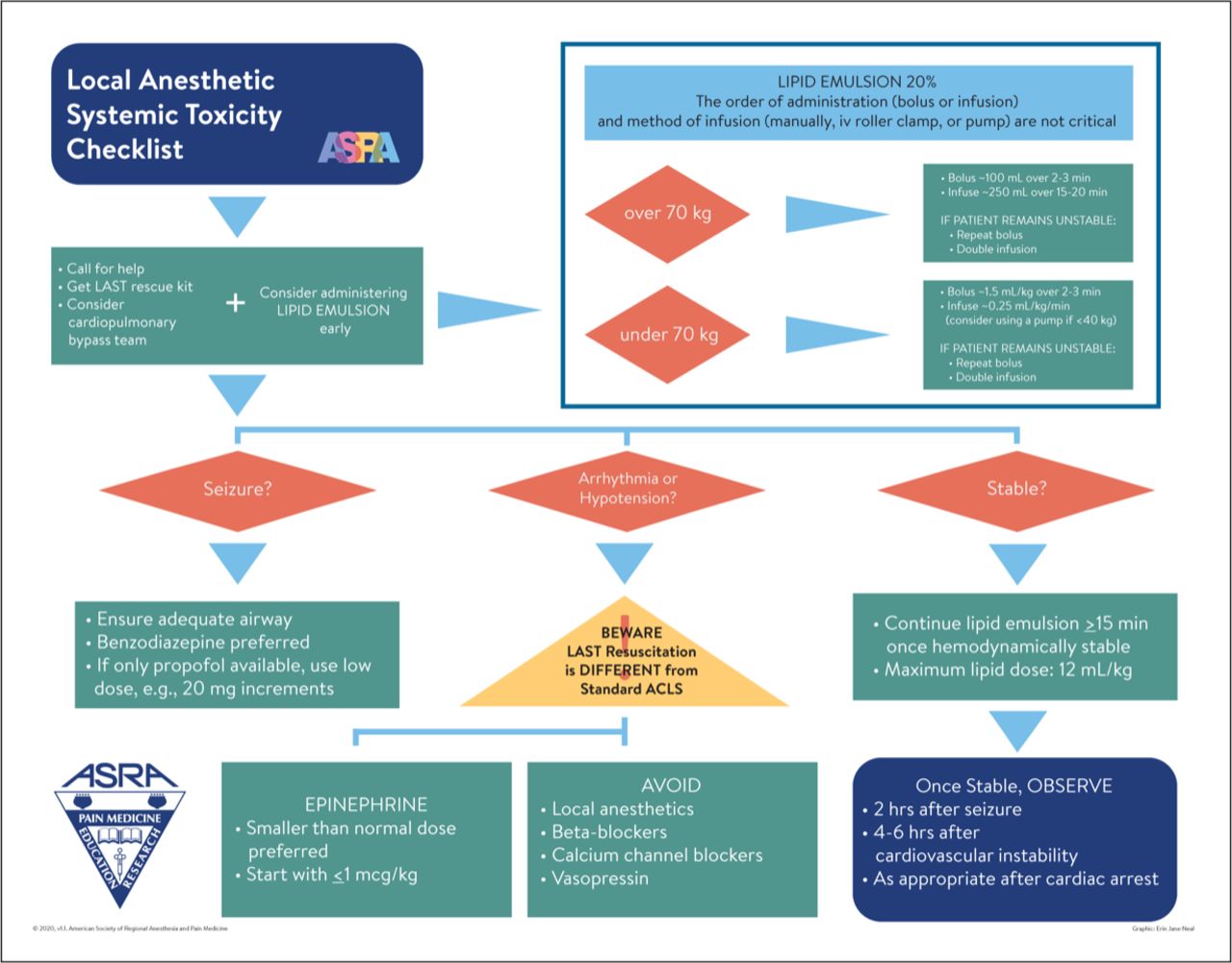
Figure 1. LAST checklist. Source: Neal JM, Neal EJ, Weinberg GL. American Society of Regional Anesthesia and Pain Medicine local anesthetic systemic toxicity checklist: 2020 version. Reg Anesth Pain Med. 2021;46(1): 81-2. PubMed. ©2020. Used with permission from the American Society of Regional Anesthesia and Pain Medicine.
References
- Neal JM, Barrington MJ, Fettiplace MR, et al. The Third American Society of Regional Anesthesia and Pain Medicine practice advisory on local anesthetic systemic toxicity: Executive Summary 2017. Reg Anesth Pain Med. 2018;43(2):113-23. PubMed
- El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018; 11:35-44. PubMed
- Gitman M, Barrington MJ. Local Anesthetic Systemic Toxicity: A Review of Recent Case Reports and Registries. Regional anesthesia and pain medicine. 2018;43(2):124-130. PubMed
- Sekimoto K, Tobe M, Saito S. Local anesthetic toxicity: acute and chronic management. Acute Med Surg. 2017;4(2):152-60. PubMed
- Rubin DS, Matsumoto MM, Weinberg G, et al. Local anesthetic systemic toxicity in total joint arthroplasty: Incidence and risk Factors in the united states from the national inpatient sample 1998-2013. Reg Anesth Pain Med. 2018;43(2):131-7. PubMed
- El-Boghdadly K, Chin KJ. Local anesthetic systemic toxicity: Continuing professional development. Can J Anaesth. 2016;63(3):330-49. PubMed
- Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med. 2013;38(4):289-99. PubMed
- Macfarlane AJR, Gitman M, Bornstein KJ, et al. Updates in our understanding of local anaesthetic systemic toxicity: a narrative review. Anaesthesia. 2021;76 Suppl 1:27-39. PubMed
- Weinberg G, Rupnik B, Aggarwal N, et al. Local anesthetic systemic toxicity (LAST) Revisited: A paradigm in evolution. Anesthesia Patient Safety Foundation. February 2020. Accessed November 1, 2022. Link
Other References
- Weinberg G. LipidRescue™️ Resuscitation. LipidRescue™️. Published 2012. Accessed March 22, 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.