Copy link
Blood Transfusion Complications
Last updated: 05/10/2023
Key Points
- Blood transfusions can lead to several complications including electrolyte derangements, hypothermia, abnormal coagulation, and possible transmission of infections.
- Platelet transfusions are the most common blood products implicated in bacterial contamination because they are stored at room temperature.
- Leukoreduction reduces the risk of cytomegalovirus transmission.
Introduction
- Blood transfusions can lead to several complications that include electrolyte derangements, acid-base abnormalities, hypothermia, and abnormal coagulation.1
- These complications occur more frequently when blood transfusions are administered rapidly in large volumes, especially during trauma resuscitation and massive transfusion.
- Transmission of infections is another important nonimmunologic complication from blood transfusions, and a frequent concern from patients.1,2
- A wide variety of organisms, including bacteria, viruses, prions, and parasites, can be transmitted through blood transfusions.1
- The rates of transfusion transmitted infections have decreased significantly due to better donor screening, sterile collection, infectious disease testing (especially nucleic acid amplification (NAT) testing), and handling processes.1
Transfusion Transmitted Infections
Bacterial Contamination
- The incidence of bacterial contamination is decreasing due to better disinfection of the venipuncture site and safer handling processes.
- The risk of bacterial contamination is around 1 in 2,500 platelet transfusions. However, clinically significant bacterial transmission occurs in about 1 in 70,000 to 118,000 platelet transfusions.2
- Platelets are required to be either tested for bacteria or undergo pathogen reduction.
- Recommendations to prevent contamination include:
- avoid donors with recent infections or fever;
- optimal product processing/handling ;
- skin disinfection before collection;
- visual inspection of the product for a darker color and gas bubbles;
- bacterial detection methods post collection; and
- pathogen reduction of the blood product.
- Platelets are the most common product implicated in bacterial contamination because they are stored at room temperature, and organisms can easily propagate at this temperature.
- Transmission of bacterial infections can rapidly lead to sepsis with fever, rigors, and cardiovascular collapse.
- Transfusion-related sepsis, while less common, can cause severe illness and death.

Table 1.
Viral Contamination
- It is rare due to donor screening and blood testing.
- Donor questionnaire should be conducted to identify high-risk behaviors for viral transmission.
- Serologic and nucleic acid testing should be done postblood collection to prevent transmission during the immunological window period.
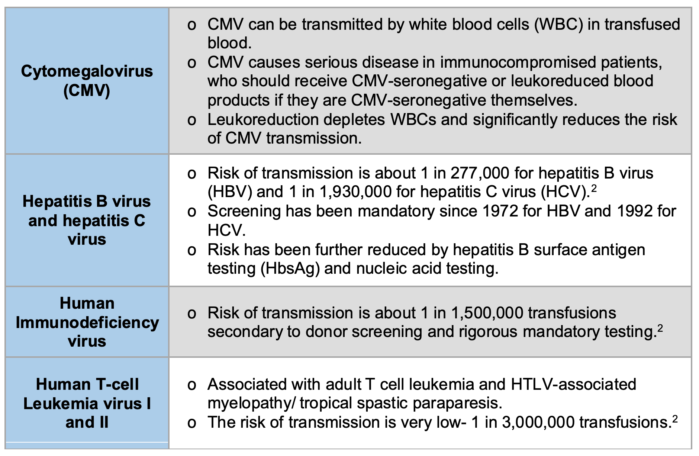
Table 2. The numbers in this table refer to the risks of viral transmission in the United States.
Other Infections
Parasites
- Malaria can be transmitted from infected RBCs in patients with latent disease. Donors are screened and deferred for 3 months if they have visited a malaria endemic country, or 3 years if they have lived in a country in which malaria is considered endemic.
Prion
- Creutzfeldt-Jakob disease is a transmissible spongiform encephalopathy. There is a theoretical risk of transmission via blood transfusion. As a result, there is a restriction of donations from family members of patients that have had a familial prion disease.
Blood Donor Screening
- In the United States, all blood for transfusion is screened for the following pathogens.3
- Hepatitis B virus
- Hepatic C virus
- HIV types 1 and 2
- HTLV types 1 and 2
- Treponema pallidum (syphilis)
- West Nile virus
- Trypanosoma cruzi (Chagas disease): first-time donors only
- CMV: only for select immunocompromised recipients
- Babesia: only in Babesia-endemic regions
- Zika virus: phased out in 2021 and no longer tested.
Massive Transfusion Complications
- Massive transfusion in adults is commonly defined as the transfusion of 10 or more units of RBCs within 24 hours or the replacement of the total blood volume in 24 hours. In children, the transfusion of more than 40 mL/kg over 6 hours is commonly used to define a massive transfusion.4
- Massive hemorrhage protocols (MHP) include the initial rapid administration of large amounts of blood products in a fixed ratio of RBCs, FFP and platelets (1:1:1). This fixed ratio can be modified when coagulation studies via blood draws or point of care systems are available. Alternatively, some centers are transfusing whole blood in situations of rapid blood loss.
- The goal is to maintain the patient hemodynamically stable while avoiding large amounts of crystalloids that can lead to dilutional coagulopathy.
- The lethal triad of trauma (hypothermia, acidosis, and coagulopathy) is a spiraling cycle associated with high mortality.5
Electrolytes Abnormalities
- Hypocalcemia: the combination of hypothermia and large amount of citrate in the transfused products causes decreased hepatic metabolism of citrate. This results in citrate toxicity and hypocalcemia.
- Calcium is important for the coagulation cascade and muscle contractility, including the myocardium.
- Hypocalcemia results in hypotension, QT prolongation, and negative inotropy.
- Hyperkalemia: occurs due to leakage of potassium from red blood cells in stored blood.
- It can result in ECG changes and lethal cardiac arrhythmias.
Hypothermia
- Rapid transfusion of blood products can cause significant hypothermia because most blood products are stored at cold temperatures.
- Anesthesiologists should use fluid warming devices and other warming techniques to maintain the patient’s body temperature.
- Hypothermia will worsen hypocalcemia and acidosis, leading to decreased production of clotting factors, eventually leading to the lethal triad of trauma.
Acid-base Abnormalities
- Metabolic acidosis
- Decreased oxygen-carrying capacity and oxygen delivery to tissues lead to intracellular ischemia and tissue hypoperfusion.
- This causes lactic acid build-up and result in metabolic acidosis.
- Metabolic alkalosis
- It can result from the administration of large amounts of citrate in blood products.
Coagulation Abnormalities
- Early trauma-induced coagulopathy
- Hypothermia, hypoperfusion, and acidosis lead to an abnormal coagulation cascade, further decreasing the ability to form blood clots.
- Dilutional coagulopathy and dilutional thrombocytopenia
- These result from transfusing large amounts of crystalloids or RBC since they do not contain any coagulation factors or platelets.
- If activating massive hemorrhage protocol (MHP), recommendations are to initially transfuse RBC, FFP, and platelets in a 1:1:1 ratio to avoid coagulopathies.
- Disseminated intravascular coagulopathy
- It can result from hemorrhage and trauma.
- It can cause consumption of platelets and coagulation factors.
- Management will include aggressive replacement of coagulation factors and platelets.
Risk Factors for Transfusion Complications
- Massive transfusion
- Rapid transfusion rates
- Platelet transfusions have the highest risk of transmitting pathogens due to storage at room temperatures.
Management of Transfusion Complications
Transfusion Transmitted Infections
- Bacterial: initial workup should include gram stain and culture (anaerobic and aerobic) of the blood product and from the patient that was transfused. Patients can quickly become septic. Management should include antibiotics and sepsis management.
- Viral: If there is a suspicion for viral transmission, the patient should be tested and started on specific antiviral medications.
Electrolytes Abnormalities
- Hyperkalemia: Hyperkalemia treatment protocol with insulin and dextrose, hyperventilation, beta adrenergic agonists (e.g., albuterol), loop diuretics (e.g. furosemide), sodium bicarbonate and calcium if ECG abnormalities are present.
- Hypocalcemia: replacement with calcium gluconate 50mg/kg if there is no central access, or calcium chloride 10mg/kg if central access is available.
Coagulopathy
- Administration of large volumes of crystalloids must be avoided.
- Hypothermia and acidosis must be avoided and aggressively treated.
- Replacement of whole blood (instead of RBCs only) must be attempted, by transfusing RBCs, FFP, and platelets in a 1:1:1 ratio.
- Coagulation tests should be performed as soon as possible, including fibrinogen, point-of-care viscoelastic testing (e.g., thromboelastography) and platelet count to direct further treatment.
- If there is ongoing intraoperative bleeding, a transfusion physician should be consulted. It may be necessary to draw another type and screen and crossmatch sample for the blood bank.
References
- Maxwell MJ, Wilson MJ. Complications of blood transfusion. Continuing Education in Anaesthesia and Critical Care. BJA Education. 2006; 6:225-9. PubMed
- Bihl F, Castelli D, Marincola F, et al. Transfusion-transmitted infections. J Transl Med. 2007; 5:25. PubMed
- Centers for Disease Control and Prevention. Blood safety basics. 2022. Accessed March 18th, 2023. Link
- Leonard JC, Josephson CD, Luther JF, et al. Life-threatening bleeding in children: A prospective observational study. Crit Care Med. 2021;49(11):1943-54. PubMed
- Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137(1):209-20. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.