Copy link
Monitoring Depth of Neuromuscular Blockade
Last updated: 04/14/2023
Key Points
- The depth of neuromuscular blockade should be monitored whenever neuromuscular blocking agents are used.
- Adequate recovery of neuromuscular blockade is defined as a train-of-four ratio (TOFR) ≥ 0.9 as measured at the adductor pollicis muscle (APM).
- Qualitative devices and clinical assessment cannot distinguish between minimal levels of residual blockade and adequate recovery.
- Only quantitative neuromuscular monitoring can detect minimal levels of residual blockade from adequate recovery.
Introduction
- The incidence of residual neuromuscular blockade (rNMB) remains unacceptably high, placing patients at risk of adverse outcomes, including hypoxia, upper airway obstruction, aspiration, prolonged postanesthesia care unit (PACU) stay, and patient discomfort.1,2
- Clinical assessment is the most common means of assessing the return of patient strength, but these tests are inaccurate.1,3
- Peripheral nerve stimulators are commonly available, but their use is not routine and they still rely on qualitative or subjective assessment of patient strength.4
- Quantitative neuromuscular monitoring devices are necessary to ensure appropriate dosing and adequate reversal of neuromuscular blocking agents.
Adequate Recovery: The problem
- rNMB (TOFR < 0.9) remains common, with reported rates as high as 64%.1,5
- rNMB has been associated with postoperative adverse events, including hypoxia, impaired hypoxic ventilatory response, impaired airway protective reflexes and increased risk of aspiration, airway obstruction, need for reintubation, increased length of PACU stay, pneumonia, atelectasis, and unpleasant symptoms of muscle weakness.1,2
- Adequate recovery from neuromuscular blockade, defined as a TOFR ≥ 0.9 measured at the APM using quantitative neuromuscular monitoring, should be confirmed prior to extubation to support patient safety.1,3
Patterns of Stimulation
- Several patterns of stimulation are used to assess a patient’s depth of blockade.
- Train-of-four (TOF) is the most common pattern of nerve stimulation and involves delivering four equal stimuli at 2-Hz.
- Train-of-four count (TOFC) is the simple count of muscle twitches resulting from TOF stimulation (range is 0/4 to 4/4) and correlates with the degree of receptor blockade.
- At a TOFC = 4, 70% of nicotinic acetylcholine receptors may still be blocked.
- Train-of-four ratio (TOFR) is determined by comparing the amplitude of the fourth to the first twitch response and is only possible with quantitative monitoring.
- Fade is present if the first twitch is evaluated as stronger than subsequent twitches.
- Fade is not detected qualitatively (by visual or tactile means) until the train-of-four ratio is below 0.4.
- Train-of-four count (TOFC) is the simple count of muscle twitches resulting from TOF stimulation (range is 0/4 to 4/4) and correlates with the degree of receptor blockade.
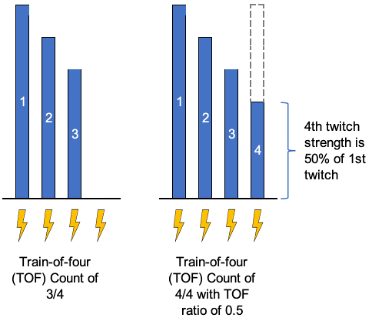
Figure 1. Train-of-four count examples. Blue bars represent the magnitude of the twitch response. Lightning bolts represent electrical stimulus from the neuromuscular monitor.
- Double-burst stimulation (DBS) delivers two 50Hz tetanic bursts spaced 750ms apart.
- It approximates the TOFR when comparing the second to the first response.
- It may increase sensitivity of qualitative assessments since fade can be detected at a TOFR around 0.6 with this method.
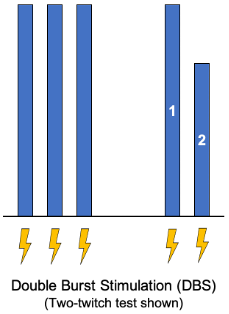
Figure 2. Double burst stimulation. Blue bars represent the magnitude of the twitch response. Lightning bolts represent electrical stimulus from the neuromuscular monitor.
- Tetany and Posttetanic Count (PTC)
- Tetanic stimulation involves a single stimulus of 50 to 100 Hz, lasting 5 seconds.
- Fade in the tetanic stimulus response is the least sensitive subjective measure of strength and is not reliably detected until the TOFR falls below 0.4.1,3
- Posttetanic potentiation can be seen in TOF measurements following a tetanic stimulus due to increased acetylcholine at the motor endplate. It is most useful when deep levels (TOFC = 0) are present.
- Posttetanic count (PTC) involves a single 5-second tetanic stimulus, followed 3 seconds later by 20 consecutive 1 Hz stimuli.
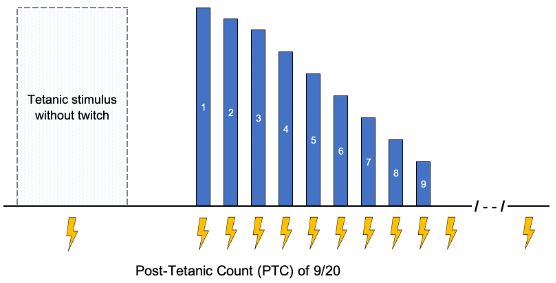
Figure 3. Posttetanic count. Blue bars represent the magnitude of the twitch response. Lightning bolts represent electrical stimulus from the neuromuscular monitor.
Depth of Blockade
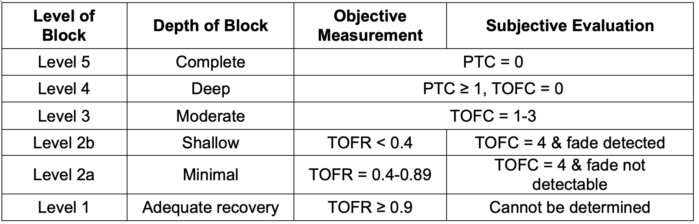
Table 1. Levels of neuromuscular blockade as determined objectively with quantitative monitoring or subjectively with qualitative devices such as a peripheral nerve stimulator (PNS) at the adductor pollicis. Note that adequate recovery cannot be determined using a PNS. Qualitative monitoring cannot distinguish minimal blockade (level 2a, TOFR 0.4-0.89) from adequate recovery (level 1, TOFR ≥ 0.9).1,3 PTC, post-tetanic count; TOFC, train-of-four count; TOFR, train-of-four ratio. Adapted from Naquib M, et al. Anesth Analg. 2018:127:71-80.3
Qualitative Assessment of Strength: Clinical Tests
- Clinical tests are commonly used to assess recovery from neuromuscular blockade, though they are imprecise and cannot accurately distinguish adequate recovery (TOFR ≥ 0.9).
- Tests including sustained head or leg lift, eye or mouth opening, and hand grip can be performed at TOF ratios between 0.4-0.9.6
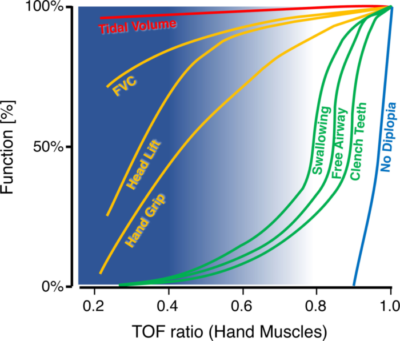
Figure 4. Average muscle-dependent functions in relation to the TOFR as measured at hand muscles. X-axis: TOFR at the APM or hypothenar muscles. Y-axis: average function as a percentage of baseline value or as a percentage of patients who passed the test. Blue background: range of TOFR in which anesthesiologists can palpate fade. Note that from a TOFR >0.4, the validity of the palpation decreases, which is illustrated by the decreasing intensity of the blue background. At TOF ratios where fade can no longer be palpated, swallowing, keeping the upper airway open, and clenching the teeth may still be impaired. FVC: forced vital capacity, TOF: train-of-four. Used with permission from Blobner M, et al. Anesth Analg 2022: 135(1):39-48.7
Qualitative Devices: Peripheral Nerve Stimulator
- Qualitative devices such as a peripheral nerve stimulator (PNS) deliver a stimulus to a nerve, and the clinician evaluates the elicited muscle response by visual or tactile means.
- PNSs are the most commonly available devices in clinical practice, but their use is inconsistent.1,4
- Their primary advantages are convenience and ease of use.
- Their critical disadvantage is the subjective nature of the evaluation, which is inaccurate at minimal levels of blockade and less accurate than quantitative monitors (QNM).
Quantitative Neuromuscular Monitoring (QNM)
- QNM is performed using devices that objectively measure muscle responses elicited from nerve stimulation.
- Unlike subjective assessment with a PNS, QNM devices can detect minimal blockade (TOFR 0.4 – 0.89) and confirm the presence of residual weakness or that adequate recovery (TOFR ≥ 0.9) has been achieved.
- QNM modalities are based on the method used to obtain measurements:1,3
- Mechanomyography (MMG) – Measures isometric muscle contraction using a force transducer.
- Kinemyography (KMG) – Assesses APM contraction by measuring the degree of bending of a sensor placed between the thumb and the first finger.
- Acceleromyography (AMG) – Based on Newton’s Second Law of Motion (i.e., force = mass x acceleration). AMG utilizes a piezoelectric sensor to measure tissue acceleration with muscle contraction (Figure 5).
- Electromyography (EMG) – Detects compound muscle action potentials at the neuromuscular junction (Figure 6).
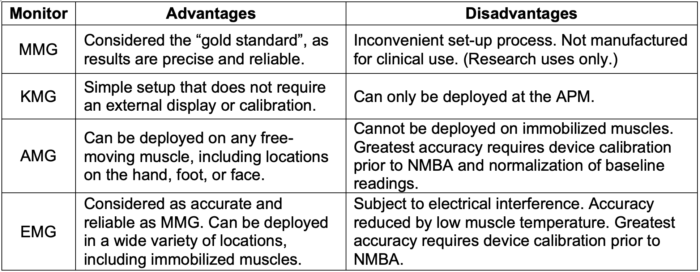
Table 2. Comparison of QNM monitor modalities.1,3
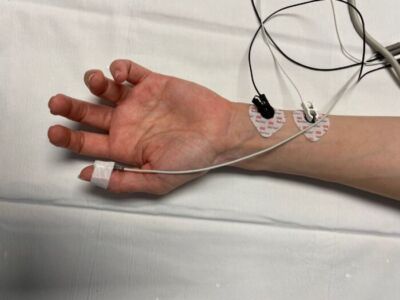
Figure 5. AMG monitoring measures tissue acceleration and requires a freely moving thumb. Pictured here is the TOF-Watch SX (Organon Teknicka BV, The Netherlands)
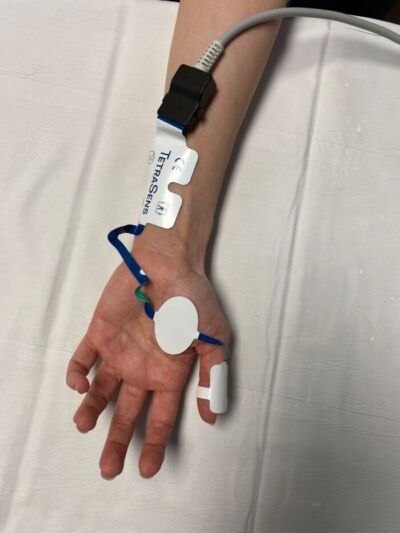
Figure 6. EMG monitoring measures compound muscle action potentials. Pictured here is the portable EMG device, the Tetragraph (Senzime AB, Uppsala, Sweden)
Monitoring Sites
- Muscle groups differ in their sensitivity to NMB agents (Table 3). Selection of the monitoring site and interpretation of the findings should take this into account.8
- The preferred location will depend on the type of monitor used and the accessibility of the site during surgery. However, adequate recovery (TOFR ≥ 0.9) is based on monitoring at the APM.1
- Differences in onset and recovery time appear to be due to differences in regional blood flow. Central muscles (diaphragm, larynx, corrugator supercilia) receive relatively high amounts of blood flow and have a faster onset and recovery. Peripheral muscles (APM and flexor hallucis brevis) receive relatively lower blood flow and have a slower onset and recovery.8
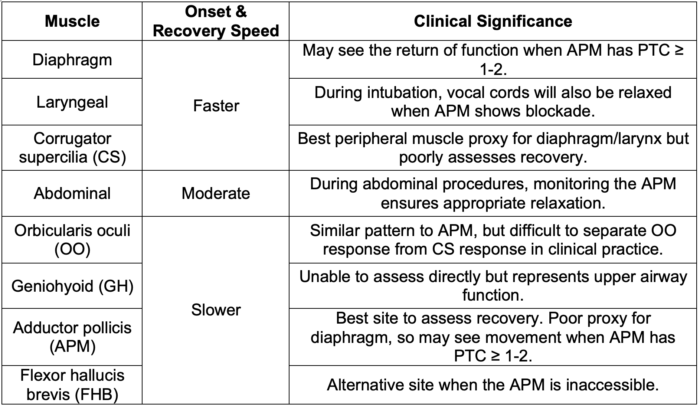
Table 3. Note that facial muscles such as corrugator supercilli (CS) may most accurately reflect optimal intubating conditions, but it recovers faster than upper airway function and will thus overestimate the degree of adequate recovery, which can place patients at risk of rNMB.
Evidence-Based Guidelines
- Based on expert evaluation of clinical evidence, the most recent practice guidelines for NMB monitoring published by the American Society of Anesthesiology (ASA) in January 2023 strongly recommend the following.1
- A TOF ratio ≥ 0.9 before extubation should be confirmed.
- Quantitative monitoring (QNM) should be used for neuromuscular monitoring.
- QNM is preferred over qualitative assessments, such as PNS.
- Clinical assessment alone should not be used.
- The adductor pollicis muscle should be used for neuromuscular monitoring.
- The eye muscles should not be used.
References
- Thilen S, Weigel W, Todd M, et al; 2023 American Society of Anesthesiologists practice guidelines for monitoring and antagonism of neuromuscular blockade: A report by the American Society of Anesthesiologists task force on neuromuscular blockade. Anesthesiology. 2023; 138:13–41. PubMed
- Murphy G, Brull S. Quantitative neuromuscular monitoring and postoperative outcomes: A narrative review. Anesthesiology. 2022;136(2):345-61. PubMed
- Naguib M, Brull SJ, Kopman AF, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127(1):71-80. PubMed
- Naguib M, Kopman AF, Lien CA, et al. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111(1):110-9. PubMed
- Saager L, Maiese EM, Bash LD, et al. Incidence, risk factors, and consequences of residual neuromuscular block in the United States: The prospective, observational, multicenter RECITE-US study. J Clin Anesth. 2019; 55:33-41. PubMed
- Kopman A, Yee P, Neuman G. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology.1997; 86:765–771. PubMed
- Blobner M, Hollmann MW, Luedi MM, et al. Pro-Con Debate: Do we need quantitative neuromuscular monitoring in the era of sugammadex? Anesth Analg. 2022;135(1):39-48. PubMed
- Renew JR. Monitoring neuromuscular blockade. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. Accessed on December 19, 2022. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.