Copy link
Injectable Corticosteroids in Pain Management
Last updated: 01/15/2024
Key Points
- Injectable corticosteroids are used in a variety of settings for pain management.
- The suggested mechanisms include anti-inflammatory effects, modulation of peripheral and spinal cord neurons, and direct stabilization of the neural membranes.
- Corticosteroid preparations that contain corticosteroid esters are particulate in nature.
- There have been several case reports of serious neurological complications following cervical and lumbar transforaminal epidural steroid injections with particulate steroids.
Introduction
- Corticosteroids are commonly used to treat inflammatory conditions. Local delivery of steroids to joints, muscles, connective tissues, and spinal structures for pain relief has gained widespread use.1
- Steroid injections can treat nociceptive articular and neuropathic radicular pain. Common indications include joint (facet, shoulder, hip, wrist, knee, ankle), bursal (subacromial, iliopsoas), epidural, transforaminal, and perineural conditions.2
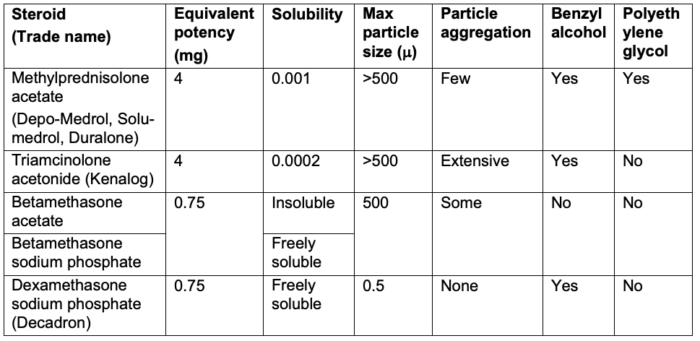
- The commonly used injectable steroids include methylprednisolone, triamcinolone, betamethasone, and dexamethasone.
- Corticosteroid preparations are either soluble or insoluble. Most corticosteroid preparations contain corticosteroid esters, which are highly insoluble in water and, thus, form microcrystalline suspensions. They require hydrolysis by cellular esterases to release the active moiety and consequently should last longer in the joint than nonester preparations. Formulations that contain corticosteroid esters have a relatively larger particulate size.2
- On the other hand, freely water-soluble preparations such as dexamethasone are clear (i.e., nonparticulate) and taken up rapidly by cells, resulting in a quicker onset and reduced duration of action.2
- In addition to the actual steroid, other chemical ingredients include preservatives (benzyl alcohol, methylparaben, sodium bisulfite) and a drug vehicle (polyethylene glycol) (Table 1).
Mechanism of Action
- Proposed mechanism of action of injectable corticosteroids include:
- Anti-inflammatory effects via inhibition of phospholipase A2 and the cyclooxygenase pathway resulting in decreased prostaglandins (Figure 1)
- Modulation of peripheral nociceptor neurons and spinal cord dorsal horn cells
- Direct stabilization of neural membranes
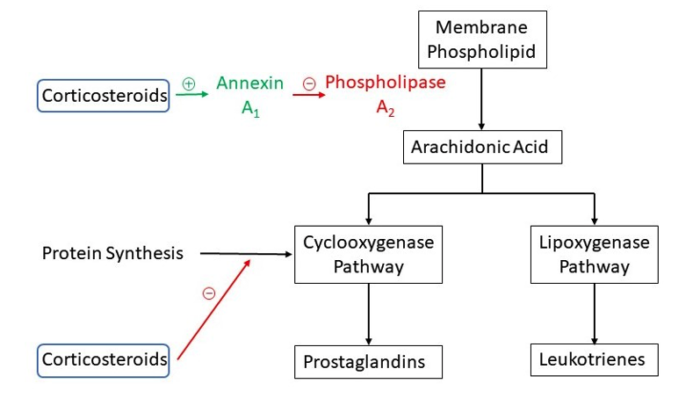
Figure 1. Mechanism of action of corticosteroids
Clinical Indications
- Indications in pain management
- Osteoarthritis
- Inflammatory arthritis
- Epidural space injections
- Peripheral nerve blockade and entrapment
- Sympathetic nerve blocks
- Connective tissue inflammation
- Muscle trigger points
- Ganglion cysts and neuromas
Adverse Effects1,2
Local Adverse Effects
- Post-injection pain
- Subcutaneous fat atrophy
- Skin depigmentation
- Soft tissue calcification
Epidural Adverse Effects
- Osteonecrosis
- Lipomatosis
Systemic Adverse Effects
- Inhibition of bone healing
- Tendon rupture
- Hyperglycemia in diabetic patients
- Articular hyaline cartilage damage
- Suppression of hypothalamic-pituitary-adrenal axis
- Poorly controlled hypertension
- Ligamentous instability
Neurological Complications After Transforaminal Epidural Steroid Injections (ESI)
- There have been several reported cases of serious neurological complications, such as brain and spinal cord infarction following cervical transforaminal corticosteroid injections and paraplegia following lumbar transforaminal injections.2,3
- Postulated causes of these serious adverse effects include2,3
- vascular injury (arterial spasm, trauma, or compression) of blood vessels supplying the spinal cord (segmental artery, deep cervical, or ascending cervical arteries);
- spinal cord or cerebral embolic infarction after particulate corticosteroid injection into an arterial vessel; and
- neurotoxicity from the preservative and/or drug vehicle in the steroid preparation.
- A comparison of the particle sizes of different steroid preparations revealed that dexamethasone and betamethasone sodium phosphate were pure liquid with no identifiable particles. Methylprednisolone acetate and triamcinolone acetonide were opaque and amorphous with large particles.3
- Based on these reports and studies, a collaborative multi-society pain workgroup made several recommendations to prevent neurological complications after ESI.4 Recommendations specific to injectable corticosteroids are:
- Particulate steroids should not be used for therapeutic cervical transforaminal ESIs.
- A nonparticulate steroid (e.g., dexamethasone) should be used for the initial injection in lumbar transforaminal ESI.
- There are situations where particulate steroids could be used for lumbar transforaminal ESIs.
- The central nervous system events have not been reported after interlaminar injections and any of the steroids can be used.
- A recent systematic review comparing the efficacy of particulate vs. nonparticulate corticosteroids in ESIs also recommends the use of nonparticulate steroids as first-line agents when performing cervical and lumbar transforaminal ESIs. There was insufficient information to make a recommendation for lumbar interlaminar ESI.5
Contraindications2
Absolute Contraindications
- Local or intraarticular sepsis
- Bacteremia
- Intraarticular fracture
- Joint Instability
Relative Contraindications
- Severe juxta-articular osteoporosis
- Coagulopathy
- Joint injection three times that year or within 6 weeks
References
- Padalia D, Shah N, Singh J, et al. Injectable corticosteroids. In: Deer TR, Pop JE, Lamer TJ, Provenzano D. Deer’s Treatment of Pain. Switzerland; Springer; 2019: 220-225.
- MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009; 252(3): 647-61. PubMed
- Benzon HT, Chew TL, McCarthy RJ, et al. Comparison of the particle sizes of different steroids and the effect of dilution. Anesthesiology. 2007; 106(2):331-8. PubMed
- Rathmell JP, Benzon HT, Dreyfuss P, et al. Safeguards to prevent neurologic complications after epidural steroid injections. Consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology. 2015; 122(5): 974-84. PubMed
- Mehta P, Syrop I, Singh JR, et al. Systematic review of the efficacy of particulate versus nonparticulate corticosteroids in epidural injections. PM R. 2017;9(5):502-12. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.