Copy link
Spinal Cord Stimulation
Last updated: 01/19/2024
Key Points
- Spinal cord stimulation (SCS) is a method for managing chronic pain that is unresponsive to surgical interventions and analgesic medications.
- Placement of a spinal cord stimulator is a two-phase procedure: a trial phase to assess pain control and device tolerance, followed by surgical implantation of a permanent spinal cord stimulator.
- SCS is minimally invasive and reversible. If the therapy is no longer desired, the device can be removed without causing permanent changes to the spinal cord.
Introduction
- Despite advances in medicine, a significant number of chronic pain patients fail to improve with conservative medical therapy. SCS is an effective treatment option for managing chronic pain that is unresponsive to analgesics and surgical interventions.
- Based on the type and frequency of electric current used and the stimulation target, there are three major types of SCS.1
- Conventional paresthesia-based SCS
- High-frequency 10 (HF 10) SCS
- Dorsal root ganglion (DRG) stimulation
- Conventional SCS involves the delivery of electric current to the dorsal columns of the spinal cord in the form of paresthesia that is achieved by percutaneous or surgical placement of electrodes in the epidural space.1 Conventional SCS uses stimulation frequencies between 40 and 200 Hz. Burst stimulation is another form of SCS that modulates the affective or medial pathway of pain, including pain vigilance and awareness.
- High-frequency SCS involves the placement of epidural leads similar to conventional SCS but uses a frequency of 10,000 Hz that does not generate the perception of paresthesias.
- DRG stimulation involves the placement of lead contacts in the neural foramen from an epidural approach in close proximity to the DRG using lower electrical currents.1 Targeting the DRG allows adequate coverage of focal and localized areas of pain, such as feet, inguinal area, groin, etc. There is some overlap in the indications for SCS and DRG stimulation, and currently, DRG stimulation is only approved for lumbosacral placement (below T10).
Indications
- SCS is most effective in treating pain with neuropathic and ischemic components. Patients with inflammatory disease or widespread myofascial pain are unlikely to benefit from SCS.1
- SCS can provide long-lasting pain relief for a wide variety of chronic pain etiologies.1
- Failed back surgery syndrome (most studied indication)
- Refractory complex regional pain syndrome
- Diabetic peripheral neuropathy
- Axial low back pain
- Refractory angina pectoris
- Phantom limb pain
- Ischemic limb pain
Mechanism of Action
Neurotransmitter Activation
- Animal studies have demonstrated that conventional SCS modulates pain through the activation of adenosine A-1 receptors and gamma-amino-butyric acid (GABA)-B receptors. Promotion of a GABA-ergic state within the basal ganglia may attenuate neuropathic pain caused by increased activity of excitatory neurotransmitters. Additionally, SCS diminishes hyperactivity of the sympathetic nervous system, leading to both antianginal and anti-ischemic effects.1
Gate Control Theory
- Although the full mechanism is not completely understood, the physiological basis for SCS can be explained by the gate control theory.2 This theory proposes the stimulation of the large, myelinated, nonnociceptive Aβ fibers of peripheral nerves causes inhibition of the small, nociceptive Aδ and C fibers via activation of inhibitory interneurons.
- Electrode leads generate electrical impulses in the epidural space and alter the electrical potential of the surrounding tissue. Because dorsal column neurons have excitable membranes, the electrical stimulus triggers action potentials. The large diameter Aβ fibers are preferentially activated, causing antidromic and orthodromic transmission of action potentials along the dorsal column.
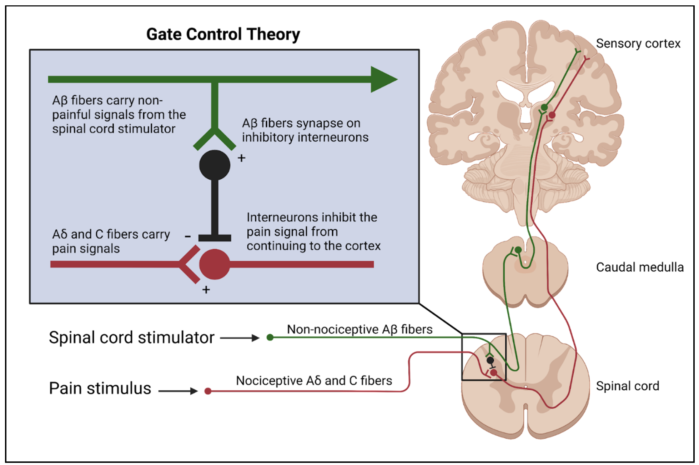
Figure 1. Inhibition of nociceptive Aδ and C fibers as a result of inhibitory interneuron activation by SCS
Hardware
- The hardware components include electrode leads connected to an implantable pulse generator (IPG) via extension wires and a portable wireless programmer.1 When implanted, the electrode leads reside in the epidural space posterior to the dorsal column of the spinal cord. The IPG serves as a battery for the device, and the external programmer allows the frequency, amplitude, and pulse width to be adjusted.
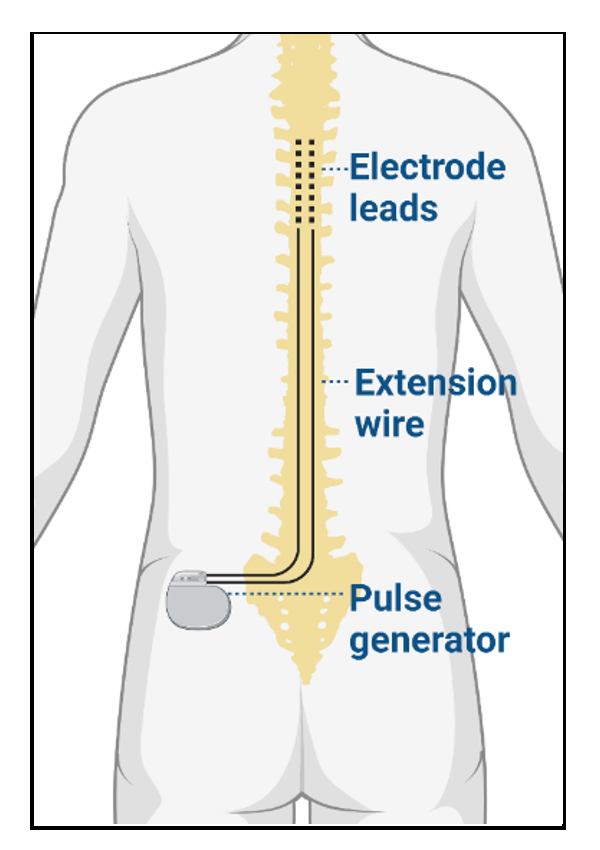
Figure 2. SCS hardware components, including electrode leads, extension wire, and pulse generator
Procedure
Preoperative Considerations
- A comprehensive preoperative evaluation must include a detailed history, physical examination, and a review of imaging studies. Additionally, a psychological evaluation is recommended to determine a potential candidate.1
- Medical comorbidities such as poorly controlled diabetes, poor cardiorespiratory health, immunosuppressed state, etc., should be optimized. Patients on anticoagulants and antiplatelet agents must hold these medications for the appropriate time period before the trial and implantation procedure.
Perioperative Management
- A trial is typically performed to gauge the efficacy of SCS prior to permanent implantation, which involves percutaneous lead placement that is powered externally for 3-7 days. Patients are considered candidates for SCS if paresthesia is achieved in the area of the patient’s pain throughout the trial period (typically 3-7 days).3
- The optimal spinal level for lead placement depends on where the pain is localized.4
- Neck: Above C3
- Shoulder: C2-C4
- Hand: C5-C6
- Low back: T9-T10
- Anterior Thigh: T11-T12
- Posterior Thigh: T11-L1
- Foot: L1
- Conventional SCS usually involves percutaneous placement of two SCS leads in the dorsal epidural space following epidural access using a Tuohy needle under fluoroscopy with minimal sedation.1 The appropriate location of the electrodes is verified by paresthesia mapping. Alternatively, an open laminectomy can be considered in place of a needle-based percutaneous approach in patients with challenging spine anatomy and is typically performed under general anesthesia with neurophysiologic monitoring.1
- The placement of HF10 SCS leads is similar to conventional SCS. However, since stimulation of those high frequencies does not generate paresthesias, no paresthesia mapping is necessary.
- DRG stimulation involves the percutaneous placement of four electrode contact leads through the neuroforamen to target the DRG following epidural access using a Tuohy needle under fluoroscopic guidance.
- Following a successful trial, the leads are tunneled subcutaneously and connected to the IPG, which is inserted into a subcutaneous pocket, typically in the flank area superior to the iliac crest, buttocks, or abdomen.
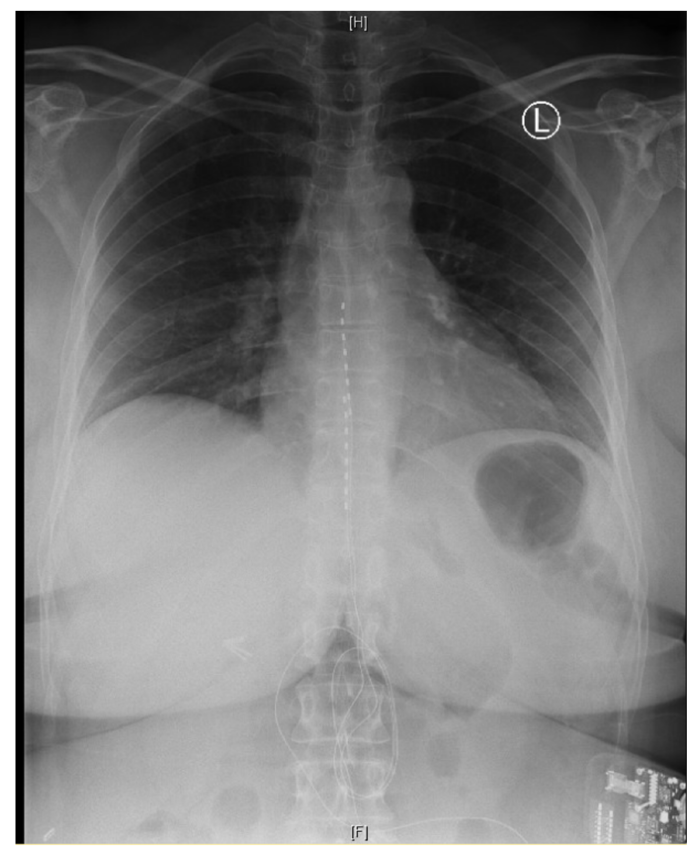
Figure 3. Chest x-ray showing the placement of two electrode leads in the epidural space
Postoperative Management
- The most common complications postoperatively are hardware-related, such as lead migration or fracture, but the incidence of such complications has decreased significantly with the discovery of new anchoring techniques.3
- Other potential complications include infection (surgical site infection, meningitis), nerve injury (transient neuropraxia, spinal cord injury), bleeding, seroma, etc.1
- Patients should take care to minimize excessive movements of the spine for 6 weeks postoperatively to minimize the chance of lead migration.
- Neurological degeneration following implantation should prompt an urgent computed tomography (CT) scan to rule out spinal epidural hematoma, a rare (~0.3%) but potentially catastrophic complication of SCS.1,5
Contraindications and Special Considerations
- Careful assessment of SCS candidates must be performed to limit adverse events. Contraindications to SCS therapy include, but are not limited to:
- Severe thrombocytopenia or uncontrolled coagulopathy
- Active infection
- Epidural scarring
- Spondylolisthesis with stenosis
- Scoliosis
- Failed psychological evaluation
- Neuraxial techniques are best avoided in patients with existing SCS. While there are several case reports describing the safe use of neuraxial anesthesia below the level of SCS leads, there is no high-level evidence to support this practice.3
- Monopolar cautery should be avoided whenever possible, and if necessary, the grounding pad should be placed as far as possible from the IPG.3
- Magnetic resonance imaging (MRI) is not an absolute contraindication, as MRI-conditional devices are now available.3 The SCS should be interrogated and switched off before entering the MRI suite and then interrogated and reactivated once the patient leaves the area. MRI can cause heating, device malfunction, image artifacts, and unintended stimulation.
- SCS should also be switched off during CT scans and lithotripsy.3
- SCS is a relative contraindication for permanent pacemakers and automated implantable cardioverter defibrillators.3 Additionally, SCS shouldn’t prevent the use of external defibrillation, cardioversion, or external pacing during cardiopulmonary resuscitation.3 Lowest energy levels should be used, and defibrillator pads should be placed as far away from the device as possible.
- Pregnancy remains a contraindication due to the use of fluoroscopy during SCS implantation. In this population, SCS therapy should be postponed until after birth.
References
- Mauck WD, Hunt CL, Olatoye OO, et al. Spinal cord and peripheral nerve stimulation for painful disorders. Adv Anesth. 2019; 37: 163-86. PubMed
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-9. PubMed
- Bull C, Baranidharan G. Spinal cord stimulators and implications for anaesthesia. BJA Educ. 2020;20(6):182-3. PubMed
- Barolat G, Massaro F, He J, et al. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg. 1993;78(2):233-9. PubMed
- Dydyk AM, Tadi P. Spinal Cord Stimulator Implant. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing. 2023.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.