Copy link
Blood Product Modifications
Last updated: 03/06/2023
Key Points
- Blood product modifications allow for the mitigation of many of the risks of blood transfusion.
- Leukoreduction reduces the risk of febrile nonhemolytic transfusion reactions, human leukocyte antigen (HLA) alloimmunization, and cytomegalovirus (CMV) transmission. It is universally performed at many facilities.1
- Irradiation is used to prevent transfusion-associated graft versus host disease.2
- Washing decreases the risk of allergic transfusion reactions and is indicated for patients with a history of severe or repeated moderate allergic transfusion reactions.3
Introduction
- Several different blood product modifications can be performed by the blood bank to decrease the risks of transfusion, including leukoreduction, irradiation, pathogen inactivation, washing, volume reduction, and aliquoting.
- Although blood product modifications can increase costs, decrease the cell yield of the product, decrease product shelf life, and/or delay blood availability, the potential benefits gained often outweigh the drawbacks when used appropriately.1
Leukoreduction
- Leukoreduction is a blood product modification that reduces the number of white blood cells in the blood product by filtration.1,4
- Leukocytes are filtered from blood products using filters of varying pore size and by electrical charge exclusion.
- Most leukoreduction is performed during or immediately after blood collection, termed pre-storage leukoreduction. Leukoreduction can also occur during storage or during the transfusion of blood products (post-storage leukoreduction, in other words, at the patient’s bedside), but this practice is rare.1
- Leukoreduction reduces the transmission of leukocyte-associated viruses, including CMV. Leukoreduced units are considered CMV-safe. This is not the same as CMV-seronegative units (where the units are tested as negative for CMV antibodies), though the risk of CMV transmission in both cases is similar.
- Leukoreduction decreases the risk of febrile nonhemolytic transfusion reactions. Removing white blood cells decreases the number of cytokines produced and contained in the blood product, and these cytokines can cause fevers in transfusion recipients. Pre-storage leukoreduction is more effective at decreasing this risk.
- Leukoreduction decreases the risk of alloimmunization to donor (HLA). By doing so, there is also a decreased risk of transfusion recipients developing antibody-mediated platelet refractoriness since platelets express class I HLA antigens.1,4,5,6,7
- Universal leukoreduction has been implemented at many hospitals in North America.1
- Drawbacks of leukoreduction include red blood cell (RBC) and platelet loss in the product and, when leukoreduction is performed post-storage, the potential for hypotensive transfusion reactions in patients taking angiotensin-converting enzyme (ACE) inhibitors.1
- Leukoreduction is not an effective measure for preventing transfusion-associated graft versus host disease.
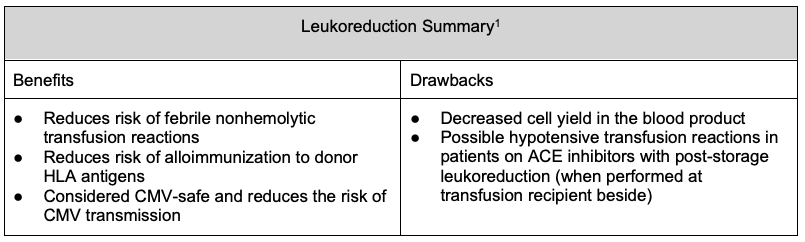
Table 1
Irradiation
- Irradiation of blood products is performed to prevent transfusion-associated graft versus host disease (TA-GVHD) by preventing the proliferation of donor T lymphocytes.2
- TA-GVHD has a mortality rate of over 90% and occurs after a cellular blood product has been transfused and donor T lymphocytes mount an immune response against the transfusion recipient.1
- In the United States, a radiation dose of 25 Gy to the midplane of the container and 15 Gy to all other parts of the container is considered adequate to completely inactivate T lymphocytes.1
- Only cellular products (RBCs, platelets, whole blood, and granulocytes) are irradiated.
- Irradiation can delay the release of blood products, decrease product shelf life, and add additional necessary security requirements for blood banks if gamma irradiators are being used (does not apply to x-ray irradiators).1
- Additionally, irradiation can increase potassium levels in blood products, with a greater risk of posttransfusion hyperkalemia, especially in neonates, patients with renal dysfunction, and recipients of massive transfusion.
- In some cases, pathogen inactivation can replace irradiation.
- Generally, irradiation is indicated for cellular blood products received by select immunocompromised individuals and for cellular blood products where the donor and recipient may have HLA similarities. Note that a recipient with human immunodeficiency virus/acquired immunodeficiency syndrome, history of solid tumors, or chemotherapy alone without another indication, except as listed below, are generally not considered indications for irradiation.
Indications for required irradiation include:1,2
- Intrauterine transfusions
- Infants younger than 4 months
- Recipients with known/suspected congenital immunodeficiencies impacting T lymphocytes
- Stem cell or bone marrow transplant recipients
- Recipients with hematologic malignancies
- Recipients on purine analogues such as fludarabine and cladribine
- Recipients of cellular components donated from a blood relative, including directed donations
- Recipients of cellular components whose donor was selected for HLA compatibility
- Crossmatched platelets
- Granulocytes
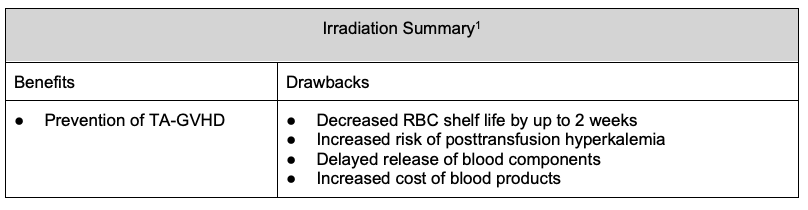
Table 2
Pathogen Inactivation
- The possibility of transmission of infectious organisms during the transfusion of blood products has long been a public health concern. Strict donor eligibility criteria and improved virus detection methods are inherently reactive and cannot eliminate the risk of transfusion-transmitted infection (TTI).8
- Pathogen inactivation, also known as pathogen reduction, refers to the treatment of blood products to reduce the transmissibility of infectious agents that may be present.4
- Pathogen inactivation technology is currently available for platelets and plasma blood components and is in development for RBCs.8
- Multiple different technologies currently exist. In the United States, solvent-detergent treated plasma (Octaplas®) and Intercept®-treated platelets and plasma are licensed Food and Drug Administration (FDA)-approved products. The latter relies on the addition of photoactive compounds to the blood component and exposure to ultraviolet (UV) light, resulting in irreversible damage to the nucleic acids of viruses, bacteria, protozoa and leukocytes, decreasing the risk of TTI.8
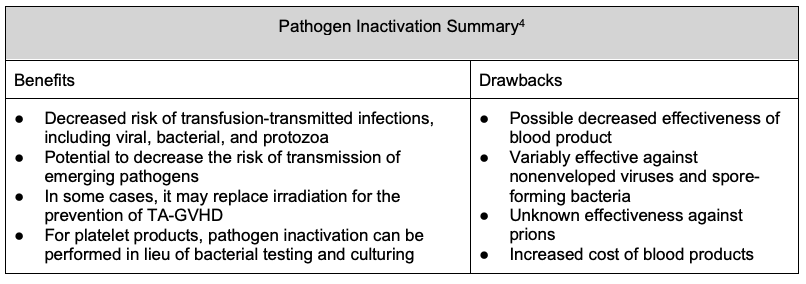
Table 3
Washing
- Washing of cellular blood components (RBCs and platelets) can be used to reduce plasma proteins, which decreases the risk of allergic transfusion reactions.
- Washing also removes immunoglobulin A (IgA), which decreases the risk of anaphylactic transfusion reactions in IgA-deficient recipients.1,3
- Indications for washing include patients with a history of severe (anaphylactic) or repeated moderate allergic transfusion reactions and patients with known IgA deficiency shown to have anti-IgA antibodies.
- Washing is performed through the addition and removal of 1-2 L of normal saline to RBCs or platelets, resulting in the loss of ~15% of the total hemoglobin and ~33% of platelets.1
- Downsides of washing cellular products include shear stress on RBCs leading to damage and platelet loss, and platelet activation resulting in decreased platelet aggregation.
- Additionally, washing is time-consuming and shortens the expiration of the product. Washed RBCs must be transfused within 24 hours of washing, and washed platelets within 4 hours.
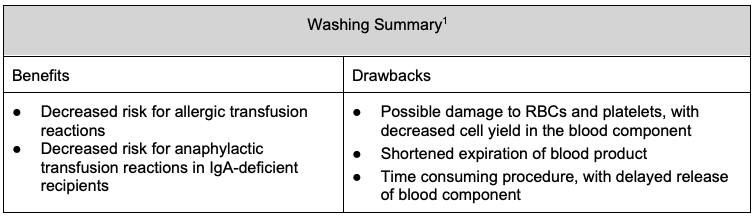
Table 4
Volume Reduction
- Transfusion can significantly increase intravascular volume. Typically, recipients tolerate the increase in intravascular volume because they either are intravascularly volume-depleted or have adequate renal function to compensate for the increase in volume.
- Patients with decreased renal or cardiac function may not tolerate the volume increase caused by transfusion and are therefore at higher risk of developing transfusion-associated circulatory overload (TACO).1
- RBCs and platelets can undergo volume reduction as both are suspended in supernatants.1
- Volume reduction is performed by centrifugation, followed by the removal of a portion of the supernatant. It is typically performed immediately before issuing a unit to a patient who may benefit from volume reduction in order to decrease the volume received.
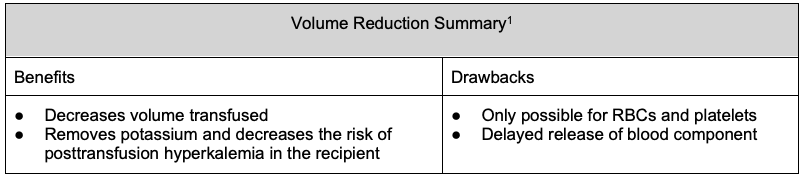
Table 5
Blood Aliquots
- Standard-sized units of blood products can be aliquoted out to yield smaller volumes for specific patient populations, most commonly pediatric patients.
- In infants who may require multiple transfusions, RBCs can be sterilely separated into 5-15 mL/kg aliquots that can be administered to patients serially over the shelf life of the RBC product. By using multiple aliquots of the same unit for same patient, the number of donor exposures is decreased.9
- A single blood product unit can be split into multiple bags (a mother bag split into daughter bags). Alternatively, aliquoting can be performed by filling syringes with the blood product from a mother unit, and syringes can be issued for transfusion instead of a bag. Aliquoting by syringes is typically done in the pediatric setting, and split bags are more commonly used for adult patients at risk of TACO.
- Blood aliquots can be used to transfuse the same volume to a recipient over a greater length of time. For example, in an adult patient at risk of TACO, issuing one RBC unit requires transfusion to be complete within 4 hours. By splitting the unit into two and issuing each half one at a time, the patient can now receive the same volume over 8 hours, allowing a slower infusion rate and decreased risk of TACO.
- Aliquots can be made in an open system or sterilely in a closed system.
- Aliquoting may shorten the expiration date of the blood component depending on the method and particular blood component.10
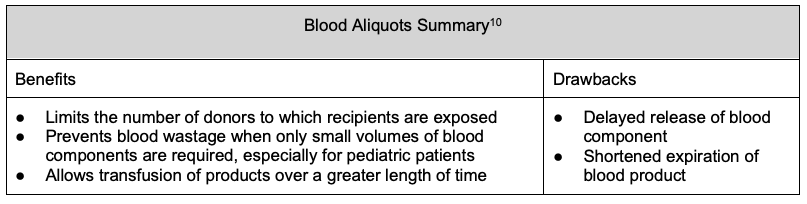
Table 6
References
- Gehrie EA, Dunbar NM. Modifications to Blood Components: When to Use them and What is the Evidence? Hematol Oncol Clin North Am. 2016;30(3):653-63. PubMed
- Dudley M, Miller R, Turnbull J. Patient Blood Management: Transfusion Therapy. In: Gropper M, ed. Miller’s Anesthesia. Ninth Edition. Philadelphia, PA: Elsevier; 2020:1546-78.
- Tobian AAR, Savage WJ, Tisch DJ, et al. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-83. PubMed
- Phou S, Kopko P. Pathogen inactivation. Pathologyoutlines.com website. Link. Accessed November 23rd, 2022.
- King KE, Shirey RS, Thoman SK, et al. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-9. PubMed
- Jackman RP, Deng X, Bolgiano D, et al. Leukoreduction and ultraviolet treatment reduce both the magnitude and the duration of the HLA antibody response. Transfusion. 2014;54(3):672-80. PubMed
- Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337(26):1861-70. PubMed
- Seltsam A. Pathogen inactivation of cellular blood products—An additional safety layer in Transfusion Medicine. Front Med. 2017;4:219. PubMed
- Luban NL. Neonatal red blood cell transfusions. Curr Opin Hematol. 2002; 9 (6): 533-6. PubMed
- Noland DK. Pediatric Transfusion Medicine. In: Jones PM, Dietzen DJ, Haymond S, Bennett MJ. eds. Pediatric Laboratory Medicine. McGraw Hill; 2017. Accessed December 23, 2022. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.